Acute lymphoblastic leukaemia (ALL) is a group of malignant haematological disorders characterised by accumulation of lymphoid cell precursors that replace normal bone marrow elements and inhibit the production of functional blood cells.1 ALL occurs in both children and adults, and more than one-half (56%) of those diagnosed are <20 years old, with chemotherapy required as standard treatment.2 The 5-year survival rate is 46% for patients aged 20–39 years, 30% for those aged 40–64, and 15% for those aged ≥65.2 Despite the increasing importance of genetic characteristics and molecular biology in ALL classification, morphological and immunophenotypic analysis remain the main techniques used in the initial diagnosis of these disorders.3,4 Immunophenotypic analysis by multiparametric flow cytometry has been improved, and it is widely used in ALL diagnosis and treatment monitoring. It allows identification and quantification of abnormal cells, classifying ALL subtypes according to cell lineage and maturation stage.5–7 In addition, the study of minimal residual disease (MRD) by multiparametric flow cytometry allows to distinguish normal lymphoid precursors from residual leukaemic cells after chemotherapy, which helps to identify the risk of relapse.8
Although several prognostic factors have been described for adult patients with ALL and outcomes have improved considerably in recent years, a significant proportion of cases still relapse. It is hoped that by pursuing a better understanding of molecular mechanisms and resistance to chemotherapy, the identification of new immunophenotypic markers may be used to guide the development of new targeted chemotherapy or immunotherapeutic agents.9–11
Since there are no clear recommendations for the use of new markers in panels for immunophenotypic diagnosis in ALL, and their influence on determining prognostic features and survival is not used in clinical practice, a systematic review was performed aiming to identify relevant publications on the influence of the new monoclonal antibodies used as immunophenotypic markers in the prognosis and survival of patients with ALL.
Methods
A systematic review was performed based on a scientific research protocol describing the aims and methods used. Within the research limitations, this synthesis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12 The question of this systematic literature review was: “Are there new immunophenotypic markers that may be used to define prognosis and survival status in acute lymphoblastic leukaemia?”
Search strategy
The literature search was conducted using PubMed, Science Direct, Web of Science, Scopus and Cochrane Library databases for articles published from 2012–2016. The investigation started in 2012 according to the last EuroFlow consortium update for ALL immunophenotyping panels.6 In addition, the reference lists of relevant papers were searched for additional ALL studies. The search consisted of a range of pertinent terms: antigens, CD (MeSH); antigens, differentiation (MeSH); biological markers (MeSH); tumor markers, biological (MeSH); prognosis (MeSH); survival rate (MeSH); survival analysis (MeSH); precursor cell-lymphoblastic leukemia/lymphoma (MeSH); and acute leukemia.
Study selection
The articles found were compared with the following defined inclusion criteria to determine the relevance of the study: (1) papers published from 2012–2016; (2) articles published in English, Spanish and Portuguese; (3) articles that used immunophenotyping by flow cytometry in their methodologies; (4) articles assessing monoclonal antibodies not included in the consensus diagnostic panels for ALL (EuroFlow consortium);13 (5) articles assessing monoclonal antibody combination, even those included separately in the consensus diagnostic panels for ALL (EuroFlow consortium);13 (6) articles assessing ALL cases; and (7) articles with available abstract and full text. Systematic and literature reviews, meta-analyses, editorials, conference proceedings and books were excluded from the study.
Two reviewers independently evaluated the titles and abstracts of the identified publications by applying the inclusion criteria. Potentially relevant articles were retrieved in full. The final inclusion of articles into this systematic review was based on agreement between both reviewers. In case of any disagreement between the two reviewers, a third reviewer inspected the full text article and finalised the decision of whether or not to use the article.
The methodological quality of each individual study was evaluated using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) assessment scale which consists of 22 items.14 High scores meant that there was sufficient information and good design. STROBE was a highly feasible and applicable method to use for evaluating systematic reviews of observational studies.
Data extraction and management
From the included studies, the following information was obtained: journal of publication, The Journal Citation Reports impact factor, location, study design, aim of the study, number of samples analysed, age, ALL classification, most incident subtype, immunophenotypic marker, cut-off value, prognostic value, gene mutation, first induction treatment protocol, follow up, survival, limitations, and STROBE scores. For inaccessible or incomplete full texts, authors were contacted for additional information.
Statistical analysis
A meta-analysis was performed for disease-free survival (DFS) and overall survival (OS) in ALL at 10, 30 and 60 months to evaluate the influence of new immunophenotypic markers on prognosis and survival. DFS is defined as the period measured from the date of achievement of a remission until the date of relapse or death from any cause. It is only used for patients that achieved complete remission. Otherwise, OS is a measure from the date of entry into a clinical trial or from the date of diagnosis to the date of death from any cause.15 The cut-off points of 10 months, 30 months, and 60 months were established due to the availability in Kaplan-Mayer curves in the studies analysed. Absence of immunophenotypic markers was considered as negative if the immunophenotypic markers had low expression, and as positive if the markers were expressed in high levels.
The statistical heterogeneity in meta-analysis was assessed using the Cochran Q test and quantified by Higgins I² test. Null hypothesis was considered when studies included into the meta-analysis were homogeneous using Cochran Q test. Heterogeneity was categorised as a scale, whereby between 0–25% was classified as no heterogeneity, and >25–50%, >50–75% and >75–100% were considered as low, moderate and high degrees, respectively.
Following the heterogeneity analysis, the model to be used in each meta-analysis (the fixed effects model or the random effects model) was chosen. The fixed effects model assumes the effect of interest is the same in all studies, and the differences observed between them are due only to sampling errors. The random effects model assumes the effect of interest is not the same in all studies, but studies that are part of the meta-analysis result in a random sample of hypothetical amount of studies. A meta-analysis was then performed using a proportion of individuals who experienced the event of interest. The meta-effect estimates of the proportion of positivity, summarised with the respective 95% confidence intervals, have been reported. The funnel graphs and regression testing of asymmetry were used to assess potential publication bias. The bias was considered significant at p=0.05. All analyses were performed by the programme R version 3.4.316 and the “Metafor” package.17
Results
The literature search
The literature search retrieved 10,209 articles. After screening titles and reviewing abstracts according to the inclusion criteria, we identified 23 potentially relevant articles that studied immunophenotypic markers and multiparametric flow cytometry (Figure 1). In the final analysis, nine studies were included in the qualitative synthesis of this review with a total of 11 markers.9,10,18–24 Two of them were used in a meta-analysis for 10-, 30- and 60-month DFS and OS.
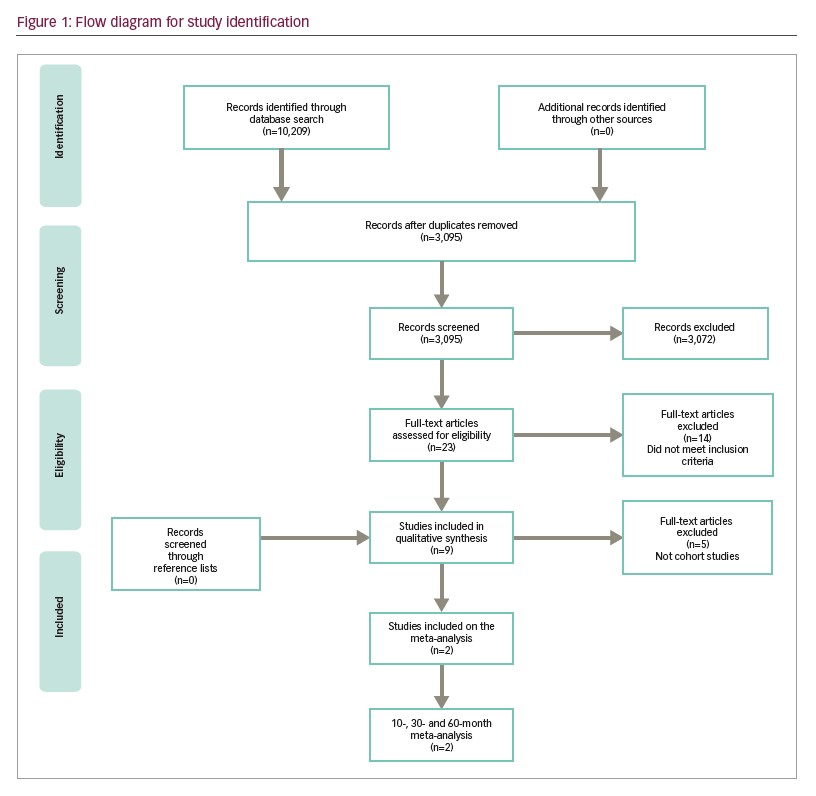
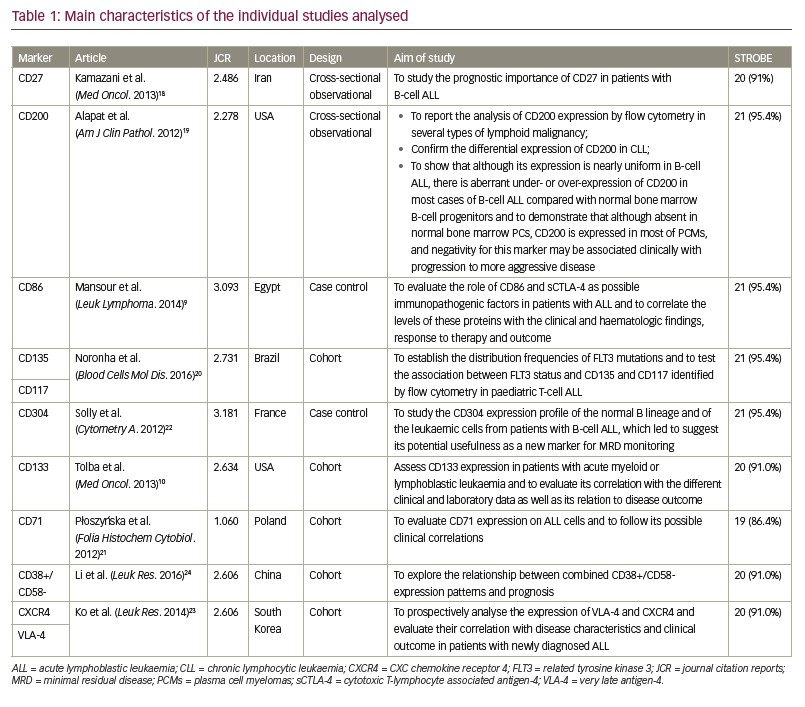
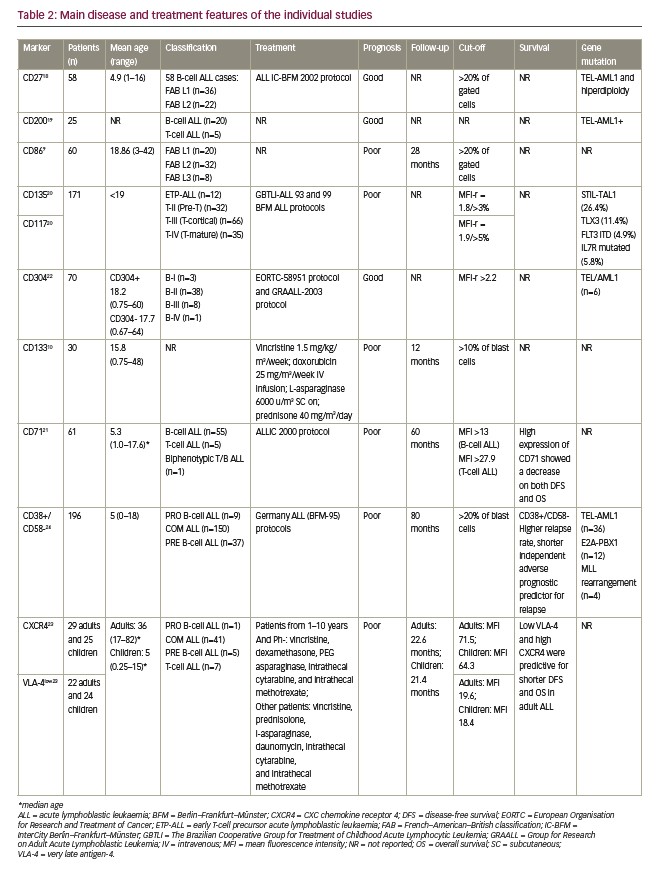
Study characteristics
Results of the nine studies included in the final analysis are shown in Tables 1 and 2. For the purposes of this study, articles published since 2012 were selected (the date of the last EuroFlow Consortium update for ALL immunophenotyping panels). In the range of these years, nine studies were included showing the inclusion and exclusion criteria was precisely applied into this study. Further, immunophenotyping data were collected at the time of patients’ diagnosis, this way there was no treatment influence on the result. This is also the reason that the date of immunotherapy initiation was available. Sample size ranged from 25–196 patients with a total of 765 haematological samples analysed.19,24 Four studies used The World Health Organization (WHO)20,22–24 diagnostic criteria and two used The French–American–British System to classify ALL cases.9,18 The other two studies classified patients only regarding B and T cells,19,21 and one did not use any system to classify them.10 The most reported subtype in the articles was common ALL followed by precursor B-cell subtype. None of the articles presented patients with Phpositive ALL.
Selected studies were from seven different countries across five continents, and the Journal Citation Reports impact factor of the journals from which the articles came ranged from 1.060–3.181 (Table 1). Patients’ age ranged from 0–82 years. A total of 556 patients were <18 years old. The expression of 11 different antigens was evaluated in all articles (Table 3). All nine studies were based on proving the impact of novel antigens on ALL prognosis while three of them were cohort studies that also ascertained the influence of these markers on patient survival.21,23,24 The most common chemotherapy regimen was based on the Berlin–Frankfurt–Münster protocol,25 although other alternative treatments were chosen by some other groups.21–23
The methodological quality of observational studies conducted with the STROBE tool showed that eight articles scored >90%, revealing a high methodological quality of the included studies. One article scored 19 points (86.4%),21 four scored 20 points (91.0%),10,18,23,24 and four scored 21 points (95.4%).9,19,20,22
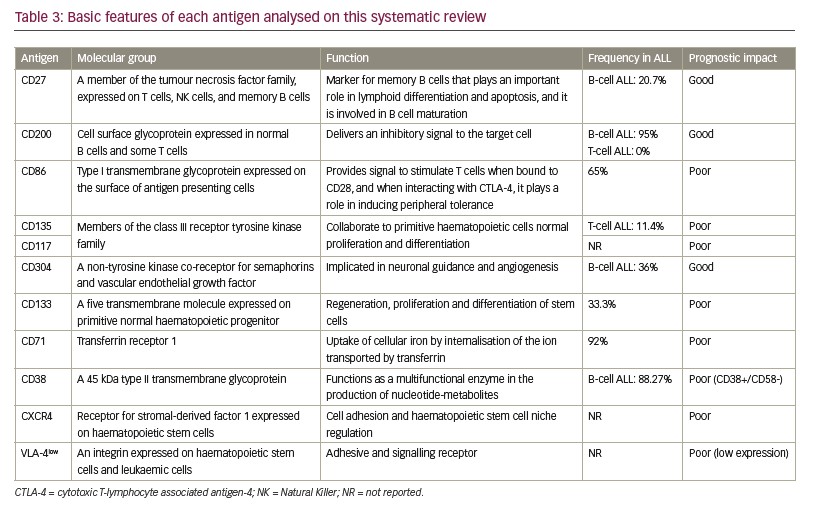
Prognostic value and survival
From the nine articles, six9,10,20,21,23,24 showed that the expression of the evaluated eight antigens has negative impact on prognosis of ALL: CD86, CD135, CD117, CD133, CD71, CXCR4, VLA-4 and CD38+/CD58–, and only three articles18,19,22 showed that expression of CD304, CD27 and CD200 has positive value on prognosis. From the five cohort studies included, three evaluated the survival in ALL with high expression of the immunophenotypic markers (Table 2).21,23,24
Regarding the poor prognostic markers, Mansour et al. showed that expression of CD86, a type I transmembrane glycoprotein expressed on the surface of antigen presenting cells, was an indicator of poor prognosis in B-cell ALL.9 Noronha et al. discoursed and analysed CD135 and CD117 in T-cell ALL, two members of the class III receptor tyrosine kinase family, showing that the combined expression of these two markers predicted TLX3, FLT3-ITD and IL7R gene mutations, and it is associated with unfavourable outcomes in adults and children.20 Tolba et al. evaluated the expression of CD133, a five transmembrane molecule expressed on primitive normal haematopoietic progenitors, in 30 patients with ALL, and found that its expression was significantly related to patients with chemotherapy resistance, as well as high incidence of relapse and death.10 Overexpression of transferrin receptor, CD71, in adult T-cell leukaemia, was associated with poor prognosis and less favourable outcomes in patients with precursor B-cell ALL, as observed by Płoszyn´ska et al.21 Ko et al. studied the expression of CXCR-4, a chemokine receptor for stromal-derived factor 1 (SDF1), and VLA-4, an integrin expressed on hematopoietic stem cells and leukemic cells in patients with ALL.23 It was verified that high expression of CXCR-4 and low expression of VLA-4 were predictive for shorter DFS, concluding that these markers are associated with poor prognosis and decreased survival. Another study performed by Li et al. investigated the co-expression of CD38+/CD58–; CD38 is a highly glycosylated member of the immunoglobulin super family, and CD58 is a type II transmembrane glycoprotein. This co-expression of CD38+/CD58– in paediatric patients with Ph-negative B-cell ALL may result in poor outcomes and an increased risk of relapse.24
Immunophenotypic markers were related to good prognosis in three studies: CD304, CD27 and CD200.18,19,22 CD304, a non-tyrosine kinase vascular endothelial growth factor (VEGF) receptor, was studied by Solly et al. They demonstrated the expression of CD304 in leukaemic blasts was more frequent in TEL-AML1-positive cases.22 TEL-AML1 fusion gene is the most frequent gene recombination in paediatric B-cell ALL, and it is associated with favourable prognosis. Although the frequency of BCR-ABL was higher in the CD304+ group (32%) compared to the CD304- group (5%), there was no significant difference. The higher expression of CD27, a member of the tumour necrosis factor family expressed on T cells, natural killer (NK) cells and memory B cells, was associated with the TEL-AML1 genotype, and it was detected in low-risk patients and in those who entered complete remission, being significantly correlated with favourable prognosis.18 The other marker, CD200, a cell surface glycoprotein expressed in normal B cells and some T cells, was present in almost all precursor B-cell ALL with aberrant over- or under-expression when compared to normal B-cell progenitors in 55% of cases. Four patients with B-cell ALL with the highest CD200 over-expression presented hyperdiploidy or were TEL-AML1 positive, these two subgroups are typically associated with an excellent clinical outcome. T-cell ALL cases were uniformly CD200 negative.19
Meta-analysis
Two cohorts were selected for meta-analysis since they demonstrated survival curves in their results, and it was possible to evaluate the DFS and OS at three different time points.21,24 The results of quantitation of two immunophenotypic markers were evaluated in these two cohorts. Homogeneity and symmetry analysis showed that both relapse and death data were progressively asymmetric and heterogeneous over the study period. This is the reason we used the fixed effects model in the relapse data at 10 months and the random effects model in all other analyses.
The OS analysis at 30 and 60 months showed a great heterogeneity with Cochran Q test (Q=6.4635; p=0.0911 and Q=32.1836; p<0.0001, respectively). The Higgins I² test showed a result of 57.38% for 30 months and 97.37% for 60 months. The same analysis was applied to DFS with Cochran Q test (Q=13.1929; p=0.0042) for 30 months and (Q=20.4940; p=0.0001) for 60 months. The Higgins I² test showed a result of 73.38% and 88.95% for 30 and 60 months, respectively. The tests were considered homogeneous for 10 months in OS and DFS, presenting HigginsI2 test of 41.61% in both evaluations. The Cochran Q test presented the same result for OS and DFS (Q=5.1487; p=0.1612).
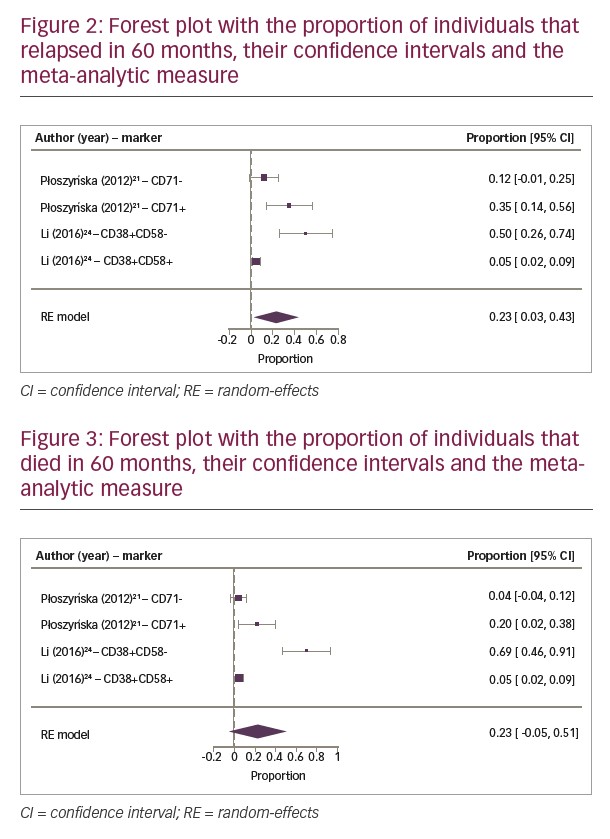
The meta-analytic results (Figures 2 and 3) show a progressive relapse rate that reaches 23% in 60 months, the only one with statistical significance, as well as the death rate that reaches 23% at the same period of time.
Discussion
Identification of new immunophenotypic markers in ALL will not only enhance our prognostic abilities but it will also allow the exploration of new therapeutic targets and MRD monitoring.26 Over the last decades, some prognostic factors have been established in ALL, including age, cytogenetic abnormalities, white blood cell count and serum lactate dehydrogenase levels.3 However, further studies are needed to improve the our understanding of leukaemia cell biology, making it possible to determine new prognostic factors that will help to improve patients’ follow-up and the therapeutic guidance. In this systematic review, eight new immunophenotypic markers were associated with worse outcomes in DFS and decreased OS.
Cell adhesion
VLA-4 and CXCR4 perform critical roles in the adhesion of hematopoietic and leukaemic stem cells to marrow stromal cells. They have important function on haematopoiesis regulation and in the immune system organisation, in addition, they also are expressed in leukaemia blast cells. The significance of CXCR4 and VLA-4 as clinical prognostic markers in leukaemia has been studied mainly in patients with acute myeloid leukaemia, and their expression has been associated with decreased DFS and OS.27 In ALL, Ko et al. verified that the association of high CXCR4 expression and low VLA-4 expression at diagnosis is correlated to worse prognosis in adults, showing shorter DFS and OS.23
Another adhesion molecule, CD58, is characterised by cytotoxic T-/NK-cell activation and immune recognition of tumour cells. CD58 is expressed on T cells, monocytes and erythrocytes while CD38 is very present during B-cell ontogeny. CD38 appears on bone marrow precursor cells but is lost on mature lymphocytes.28 Li and collaborators found that the CD38+/CD58- phenotype in patients with ALL is implicated in disease course, leading to a decrease on OS and DFS. In addition, CD38+/CD58- phenotype has been considered as an independent adverse prognostic factor that can be identified in high-risk patients at diagnosis. Otherwise, an increase in OS and DFS was identified in patients with ALL with CD38+/CD58+ expression, resulting in a better outcome.24
Programmed cell death
CD86 is a protein expressed on antigen presenting cells that deliveries costimulatory signals essential to T-cell activation and survival. CD86 acts on apoptosis regulation, and its high expression has been associated with leukaemic blast cell survival by programmed cell death inhibition. A decrease in OS was found in patients with ALL that express high levels of CD86. On the other hand, lower levels of this marker were associated with less relapse or death.9
CD27, a memory B cell marker, participates in lymphoid differentiation and maturation, beyond this, it plays an important role in apoptosis as a cell-death regulator. Kamazani et al. showed in their study that higher expression of CD27 was detected in low-risk patients and in those who entered complete remission, being significantly correlated with favourable prognosis.18
Proliferation and differentiation
CD133 expression is associated with regeneration, proliferation and differentiation of stem cells. Studies revealed that CD133 is a primitive normal haematopoietic progenitor marker, more specific than CD34. Patients with ALL with CD133 expression on blast cells present a more immature phenotype, and they are associated with an unfavourable prognosis.29,30 A meta-analysis conducted by our research group showed that CD133 expression was also associated with decreased OS, being considered as a poor prognostic factor in acute myeloid leukaemia.10,31 It was demonstrated by Tolba et al. that the expression of CD133 in ALL cases also has a negative impact leading to higher resistance to standard chemotherapy and higher relapse and death rates. This way, CD133 expression is an independent prognostic factor associated with poor prognosis in patients with ALL.10
Noronha et al. discoursed and analysed CD135 and CD117, receptors highly expressed in lymphoid and myeloid early cell populations that promote the growth and differentiation of primitive haematopoietic cells.20 CD117 was found with higher frequency than CD135, and the co-expression of these two markers can predict FLT-ITD mutation. Although FLT-ITD mutation occurs at low frequency in children with T-cell ALL, it is possible to predict it by multiparametric flow cytometry analysis of these two markers in the panel of monoclonal antibodies. This makes it possible to screen and identify patients who would benefit from treatment with inhibitors of tyrosine kinase.20
Markers with other functions
CD304 plays an important role in various biological processes, including angiogenesis, binding to VEGF, axonal guidance, tumorigenesis and immunologic response.32 Solly and collaborators identified this marker as a promising target for anti-leukaemic and antiangiogenic treatment strategies in patients with leukaemia, and as an MRD marker that could be applicable in a large subset of patients with B-cell ALL. The better understanding of the biological function of CD304 can include it as a prognostic factor or therapeutic target.22 Other groups have confirmed the overexpression of CD304 as an important MRD marker.33,34 More recently, the EuroFlow Consortium added CD304 antigen in B-cell ALL MRD evaluation by next-generation flow cytometry.35
CD71 is highly expressed on the surface of cells of the erythroid lineage and weakly expressed in ALL and diffuse large B-cell lymphoma.36 CD71 was included in a study performed by Płoszyn´ska et al., and it was demonstrated that CD71 was present on lymphoblasts of almost all cases of ALL. Its expression was statistically higher on T-lineage ALL than B-lineage ALL. Despite no statistical difference found, it was observed that the DFS and OS were shorter in patients with B-lineage ALL with CD71 over-expression.21
Interaction between CD200–CD200R acts on regulation of immune responses, myeloid differentiation and/or activation, foetal loss and transplant rejection.37 CD200 suppresses the myeloid-cell functions, such as neutrophils and macrophages, by binding to the CD200 receptor and stimulating differentiation into the regulatory T-cell subset.38 Alapat and collaborators conducted a study that showed CD200 expression is associated with precursor lymphoid malignancies, being negative in most T-cell ALL cases and positive in about 95% of B-cell ALL cases. It was reported in this study that hyperdiploidy and TEL-AML1, genetic alterations that are typically associated with an excellent clinical outcome, were present in all B-cell ALL cases when CD200 over-expression was observed, making it a potential therapeutic target.19 Clinical trials using samalizumab, a humanised monoclonal antibody that blocks CD200, are been conducted in patients with advanced solid tumours and acute myeloid leukaemia.39
Regarding the meta-analytic estimate, immunophenotypic markers are not related to each other, since their pathogenicity is not necessarily associated; making them independent prognostic factors. A forest plot was conducted with the relative risks and their confidence intervals from the relapse and death rates. This demonstrated that Płoszyn´ska’s study presented the highest sample number, and consequently the highest number of events.21 The studies of Płoszyn´ska et al.21 and Li et al.24 presented significant differences between both studies, observed by a shift to the right on the horizontal line that represents 95% confidence interval, and the vertical line that represents the relative risks (Figures 2 and 3). Despite high heterogeneity, this analysis showed an increasing relapse rate of 23% at 60 months. Analysis of the individual studies did not reach statistical relevance, however after compiling the results of the two studies, the meta-analysis, represented by the diamond on the forest plot, proved that null hypothesis is not true, confirming that CD71+ and CD38+/CD58- phenotypes are associated to increased relapse and death risks. The diamond’s shift to the right and close to number 1 showed a positive correlation in which the presence of CD71 and absence of CD58 expression (CD38+/CD58-) is associated with a relative risk of relapse and death rates (Figures 2 and 3).
In the first 2 years, DFS showed a progressive relapse when there was the expression of CD71+ and CD38+/CD58-, but only at 60 months did CD38+/CD58- show a significant difference. Nonetheless, CD71+ expression in OS analysis was not proportional to relapse since it was observed that there was response to treatment after relapse, maintaining mortality rates lower than relapse rates. On the other hand, in the first and third years, the expression of CD38+/CD58- also resulted in no significant increase in mortality rate, but the number of relapses was proportional to deaths. In addition, the mortality rate at 60 months was two-times greater than the survival rate.
Through comparative analysis of these two markers, the CD38+/CD58- phenotype was more associated with unfavourable prognosis than CD71+, being related to low OS at 60 months. Thus, CD38+/CD58- phenotype is a medium-term predictor of mortality for patients with ALL.
Strengths and limitations
The strengths of this analysis include a wide search covering more than 10,000 articles; applying stringent criteria that resulted in few relevant articles; comprising populations of different races/ethnicities from a wide geographic range; journals with high impact factors; and STROBE scores of >90% for almost all articles, revealing a high methodological quality. The study also has limitations regarding criteria to determine positive or negative antigen expression that varied across the included studies and the low number of cases in some studies. Survival in formation was extracted from survival curves and not from a mortality table. The search only included articles published in English, Spanish and Portuguese, and may have missed relevant publications in
other languages.
Conclusion
In conclusion, this study provides information regarding the independent prognostic factors that could be included into clinical protocols, aiding risk stratification and therapeutic guidance for ALL. The meta-analysis revealed that expression of CD38+/CD58- incurs an increased relapse rate of 23% at 60 months. This finding led to the conclusion that the CD38+/CD58- phenotype can be considered a predictor of mortality for patients with ALL. More studies are needed to confirm these findings.












