The development of targeted therapies has resulted in a new paradigm in the treatment of anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC), and ALK tyrosine kinase inhibitors (TKIs) are the standard of care in ALK-positive NSCLC. However, ALK inhibitors also present new challenges. As a result of improved outcomes, patients will be receiving these agents for long periods of time. Furthermore, these drugs often target multiple pathways and may cause a range of toxicities. Since ALK inhibitors will be used by a multidisciplinary team in a specific subgroup of patients, physicians are likely to have limited experience in their use.sup>1 It is therefore essential that physicians and caregivers are aware of treatment-related adverse events (AEs) in order to optimise their management and thus enhance the patient’s quality of life. This article will focus on the management of AEs in ALK-positive NSCLC receiving targeted therapy.
The Role of ALK Signalling in NSCLC and the Use of Tyrosine Kinase Inhibitors
NSCLC is characterised by extensive genomic instability, which results in dramatic functional changes and is the molecular basis of lung carcinogenesis.2,3 Among the most frequently encountered targetable genetic alterations are chromosomal rearrangements of the ALK gene, which encodes a receptor tyrosine kinase. The ALK association with NSCLC was reported in 2007, following the discovery of a small inversion within chromosome 2p that juxtaposes the 5′ end of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the 3′ end of the ALK gene, resulting in the novel fusion oncogene EML4-ALK in NSCLC cells.4 ALK-rearranged tumours depend on ALK for growth and survival and are therefore sensitive to ALK inhibitors.4,5 ALK-positive NSCLC accounts for 4–5 % of adenocarcinomas of the lung,6 and is more common in males than in females.7 The majority of cases of ALK rearrangement arise in patients with no smoking history or light smokers.8,9
ALK inhibitors have shown dramatic and sustained responses in clinical studies. The first-in-class crizotinib (Xalkori®, Pfizer) is an oral, small-molecule TKI that targets ALK, MET and ROS1,10 and received accelerated approval from the US Food and Drug Administration (FDA) in 2011.11 Since its launch, crizotinib has become the standard of care in ALK-positive NSCLC. However, disease typically relapses
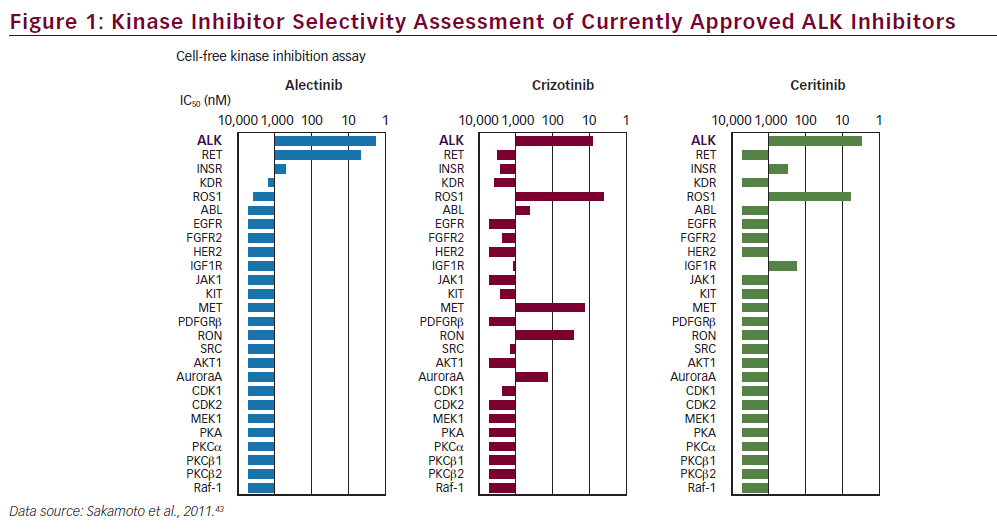
within a year because of the development of drug resistance.12,13 As a consequence, second-generation ALK inhibitors have been developed with increased potency and potential to overcome acquired resistance to crizotinib, notably being characterised by a broader activity encompassing several secondary mutated resistant ALK proteins, including ceritinib and alectinib. In 2014, ceritinib (Zykadia®, Novartis), an oral, small-molecule, ATP-competitive, TKI of ALK,14 received FDA Breakthrough Therapy designation for the second-line treatment of ALK-positive NSCLC following a clinical study of patients with metastatic ALK-positive NSCLC.15 Unlike crizotinib, ceritinib does not inhibit the kinase activity of MET, but inhibits the insulin-like growth factor 1 (IGF-1) receptor as well as ROS1 (see Figure 1).16 A novel ALK inhibitor, alectinib (Roche/Chugai), also selectively inhibits ALK with a high affinity, as well as RET.17 Alectinib (Alecensa®, Roche, Chugai) is approved in Japan and has received FDA Breakthrough Therapy designation. Another promising ALK inhibitor, AP26113 (Brigatinib®, Ariad), has also received Breakthrough Therapy designation by the FDA following the results of a phase I/II trial.18
Other second-generation ALK inhibitors are in early-stage clinical development, including X-396 (Xcovery);19 PF-06463922 (Pfizer),20 ASP302621 and TSR-011 (Tesaro).22
Clinical Trial Data Describing the Efficacy and Safety of ALK Inhibitors
In two phase III studies, crizotinib was found to be superior to first- and second-line standard chemotherapy in patients with previously treated, advanced ALK-positive NSCLC.23,24 The most common AEs associated with crizotinib include visual disorders, gastrointestinal effects (nausea, diarrhoea, vomiting, constipation), oedema and fatigue (see Table 1).11 Among 497 patients for whom data are listed in the prescribing information, deaths within 28 days of the last dose of study drug occurred in 45 patients. Ten (2.5 %) patients died within 28 days of their first dose of study drug. Causes of death included disease progression (32 patients), respiratory events (9) and other (4). Respiratory causes of death included pneumonia (2), hypoxia (2), acute respiratory distress syndrome (ARDS) (1), dyspnoea (1), pneumonitis (1), empyema (1) and pulmonary haemorrhage (1). Other causes of deaths included septic

shock, disseminated intravascular coagulopathy (DIC), cardiovascular event and death due to unknown cause (1 each).11 Rates of AEs were similar in the recent study, in which crizotinib was used in the first-line setting.24 Following prolonged treatment with crizotinib, new AEs have not emerged.25 The recommended dose of crizotinib is 250 mg orally, twice daily until disease progression or no longer tolerated by the patient. In patients with severe renal impairment (creatinine clearance <30 ml/ minute) not requiring dialysis, the dosage is 250 mg orally, once daily.
The safety evaluation of ceritinib is based on data from the ASCEND 1 (A Dose Escalation/Expansion Study of LDK378 in Patients With Tumors Characterized by Genetic Abnormalities in Anaplastic Lymphoma Kinase) phase I study of ceritinib (n=255).26 Dose reductions due to AEs were
reported in 59 % of patients. The most frequent AEs, reported in at least 10 % of patients, which led to dose reductions or interruptions are listed in Table 1. Fatal adverse reactions occurred in 5 % of patients. These comprised: pneumonia (four patients), respiratory failure, interstitial lung disease (ILD)/pneumonitis, pneumothorax, gastric haemorrhage, general physical health deterioration, pulmonary tuberculosis, cardiac tamponade and sepsis (one patient each). Discontinuation of therapy due to AEs occurred in 10 % of patients. The most frequent AEs leading to discontinuation in 1 % or more of patients were pneumonia, ILD/ pneumonitis and decreased appetite.27
In a phase I/II trial, among patients who progressed on or were intolerant to crizotinib, alectinib achieved an overall response rate (ORR) of 55 %, including patients with central nervous system (CNS) metastases and good tolerability in crizotinib pre-treated NSCLC patients.17,28,29 Recent phase II clinical trial data (NP28673 study) show that alectinib may be effective in ALK-positive NSCLC patients who had progressed on crizotinib; most had also failed prior chemotherapy and had CNS metastases.30,31 Dose reductions due to AEs occurred in 8.7 % of patients, dose interruptions due to AEs occurred in 19.65 of patients and drug withdrawals due to AEs occurred in 8.0 % of patients.
The subsequent discussion will focus on managing AEs associated with crizotinib, ceritinib and alectinib (see Table 1) since, at the time of writing, these are the only three ALK inhibitors to have received regulatory approval.
Management of Adverse Events Associated with the Use of ALK Inhibitors
In clinical trials, crizotinib, ceritinib and alectinib were all relatively well tolerated. While the possibility of serious AEs from currently available

ALK inhibitors is relatively low, there is a need for regular monitoring to mitigate the risk. It is therefore essential to educate physicians on strategies to manage the AEs associated with these drugs.
Gastrointestinal Effects
Gastrointestinal effects are the most common AEs associated with crizotinib and ceritinib, and have also been reported in alectinib (see Table 1). Taking these agents with meals has been shown to reduce nausea in some patients.25 Anti-emetics such as metoclopramide or dimenhydrinate may be prescribed.32 Prochlorperazine and 5-HT3 receptor antagonists, such as ondansetron, should be avoided with crizotinib and ceritinib because of the risk of QT prolongation.32 Diarrhoea should be managed by dietary modification, hydration and, in severe cases, the use of a medication such as loperamide.32 Constipation should be managed using laxatives and dietary modification.32 In severe cases, dose modification may be considered.27 Dyseugia, including a heightened taste for sweet and sour but diminished taste for hot and spicy, has also been reported with crizotinib7,24 and alectinib;33 however, a recent report describes a case of crizotinib-induced dysgeusia that was resolved by switching to alectinib.34
Hepatotoxicity
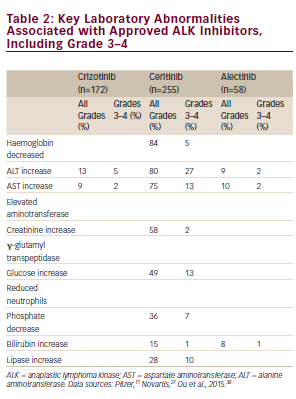
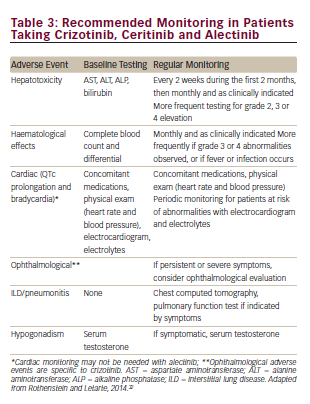
Since crizotinib, ceritinib and alectinib are metabolised primarily in the liver, hepatic impairment can result in increased drug concentrations. These agents should be used with caution in patients with significant hepatic dysfunction. Abnormalities in liver enzymes have been reported in clinical trials of crizotinib, ceritinib and alectinib.11,17,27,35 Patients taking crizotinib, ceritinib and alectinib should be monitored with laboratory tests including alanine transaminase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase and total bilirubin once a month and as clinically indicated, with more frequent testing in patients who develop elevations of liver enzymes (see Table 3), particularly during the first 2 months of treatment, when most elevations of liver enzymes are reported. Abnormal liver tests were reversible and reduced to baseline in the majority of patients with dose modification. Based on the results, dose modification, temporary or permanent drug discontinuation may be considered (see Table 4).11,27 In the case of impaired reduced hepatic function, patients should be advised to report any symptoms that may indicate hepatic injury: fatigue, jaundice, loss of appetite, dark urine, pruritus, nausea or vomiting, hypochondrial pain and bleeding or bruising more easily than normal.27,33
Cardiac Effects
Cardiac AEs, primarily bradycardia and QT elevation, have been reported with crizotinib and ceritinib but not with alectinib, though the number of treated patients with alectinib is small.11,27 It has been suggested that all patients should undergo cardiac evaluation before starting ALK-inhibitor therapy. This should include: heart rate, blood pressure, electrocardiography and electrolyte monitoring (specifically potassium, calcium and magnesium).32 These parameters should be monitored at intervals determined by the patient’s cardiovascular risk profile. Patients should be advised to report any new chest pain or discomfort, dizziness, lightheadedness, fainting or abnormal heartbeats.27 It is important to regularly update the list of patient’s concomitant medications. Concomitant use of ALK inhibitors and agents known to cause bradycardia (e.g. beta-blockers, non-dihydropyridine calcium channel blockers, clonidine and digoxin) or QT prolongation should be avoided.27,32 If co-administration cannot be avoided, dose reduction of ALK inhibitors may be considered. Crizotinib and ceritinib should be avoided in patients with congenital long QT syndrome. In cases of nonlife- threatening symptomatic bradycardia that is not life threatening, ALK inhibitors should be discontinued until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, after which dose modification may be considered. Electrolyte abnormalities (specifically potassium, calcium and magnesium) should be corrected.27,3
Haematological Effects
Grade 3 or 4 lymphopenia, neutropenia and thrombocytopenia has been reported with crizotinib,11 and neutrophenia with alectinib (see Table 1).17 If a patient experiences grade 3 or 4 haematological toxicity, the ALK inhibitor should be discontinued until complete blood cell counts return to grade ≤2. According to the crizotinib prescribing information, in cases of grade 4 myelosuppression with crizotinib, the dose should be reduced to 200 mg twice daily when treatment is resumed. In the case of recurrent haematological toxicity, crizotinib should be withheld again until recovery to grade ≤2, then resumed at 250 mg once daily. Crizotinib should be permanently discontinued in the event of further grade 4 recurrence.11
Pulmonary Effects
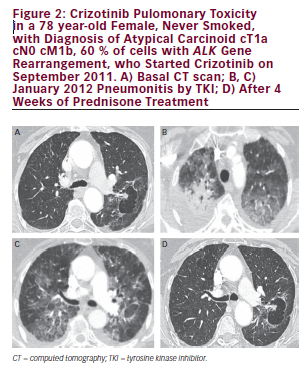
In patients with unexpected lung symptoms, such as dyspnoea, fever, cough with or without mucous or chest pain, ILD and pneumonitis should be considered and ALK inhibitors discontinued.11,27,35 Due to the high incidence of pulmonary disease at baseline, it can be difficult to diagnose drug-associated pneumonitis and ILD. Chest computed tomography (CT) examination and, if indicated, pulmonary function test should be performed. Treatment should be discontinued if ILD or pneumonitis is confirmed. Preliminary data suggest that the addition of steroid medication enables retreatment with crizotinib, but further studies are required to confirm this.37 At the time of writing, no data were available for other ALK inhibitors.
Ophthalmological Effects
Visual disturbance is an AE to crizotinib,11 although rare cases of blurred vision have been reported with ceritinib27 and alectinib.33 Events usually happen early (median time to onset <2 weeks), are generally short lived, are not associated with clinically meaningful changes at ophthalmological assessment and have a minimal impact on activities of daily living.38 Patients are advised to refrain from driving or operating machinery if they experience visual disturbance.11 If these events worsen in severity, ophthalmic assessment should be considered. Rare cases of optic neuropathy and blindness have been reported following administration of crizotinib,39 and this AE warrants further investigation.
Blood Glucose Effects
Hyperglycaemia may occur in patients taking ceritinib. It is therefore important to monitor serum glucose levels and initiate or optimise anti-hyperglycaemic medications as needed. If the drug effect is severe (persistent hyperglycaemia >250 mg/dl despite optimal antihyperglycaemic therapy), ceritinib should be discontinued until glycemic control is achieved, then resumed with a 150 mg dose reduction If adequate hyperglycaemic control cannot be achieved with optimal medical management, ceritinib should be discontinued.27
Other Effects
Hypophosphataemia has been reported in a small proportion of patients taking ALK inhibitors. In severe cases, parenteral phosphate may be used but this is much more dangerous and requires monitoring of calcium, phosphate and electrolyte levels every 6 hours as response is unpredictable, and should only be used when serum phosphate levels are under 1.5 mmol/l.40
Hypogonadism is a frequent and undiagnosed occurrence in advanced cancer patients,41 and can be particularly distressing for NSCLC patients who may be relatively young and likely to be on therapy for a long time. Symptoms of androgen deficiency and reduced testosterone levels have been reported in patients taking crizotinib: in a study of 32 crizotinibtreated and 19 non-crizotinib treated patients, mean total testosterone levels were 25 % below the lower limit of normal (LLN) in 84 % of crizotinib-treated patients compared with 29 % above LLN in 32 % of the non-crizotinib-treated patients; p=0.0012. Five of nine patients (55 %) with low testosterone were given testosterone supplementation and subsequently showed in symptoms, together with increases in testosterone above LLN..42 It is therefore advisable to monitor testosterone levels at baseline and at regular intervals in male patients.11
Peripheral oedema has been reported in patients taking crizotinib (grade 1–2, up to a third of patients)23–25 and alectinib (15 % grade 1–2; 2 % grade 3).17 No specific recommendations have been made for management of this AE, but conservative management strategies, such as leg elevation, compression stockings and dietary salt modification, may be considered.32
ALK Inhibitors During Pregnancy
There are no adequate and well-controlled studies in pregnant women using ALK inhibitors; however, non-clinical studies indicate that these agents may cause foetal harm when administered to pregnant women and crizotinib and ceritinib have been placed in FDA pregnancy category D.11,27 It is also advised to avoid pregnancy while taking alectinib.35 Breastfeeding is not recommended during use of these agents.
Drug Interactions
Crizotinib, ceritinib and alectinib are metabolised primarily by CYP3A4.11,27 Concurrent use of these agents with strong CYP3A inhibitors and strong CYP3A inducers should be avoided. If concurrent use of a strong CYP3A inhibitor is unavoidable, crizotinib and ceritinib should be reduced by one-third. Patients should avoid grapefruit and grapefruit juice as these are strong CYP3A4 inhibitors.27
Patient and Physician Education
The key to successful management of patients taking ALK inhibitors is careful patient selection before drug administration, followed by education of both physician and patient. The prescribing physician should be familiar with the package insert contents.33 Finally, regular patient surveillance, in terms of blood tests and concomitant drug use, is essential.
Summary and Concluding Remarks
ALK inhibitors represent a new treatment paradigm in NSCLC, offering improved outcomes compared with cytotoxic chemotherapy.
However, these agents target multiple pathways and are therefore associated with a wide range of AEs, including gastrointestinal AEs, and hepatotoxicity. While the majority of AEs are reversible, manageable and not severe, it is important that both physician and patients are aware of toxicities to ensure prompt treatment. There is a need for proactive treatment of the first symptoms to prevent more severe AEs. There is also a need for further studies to fully elucidate the differences in AE profiles between different ALK inhibitors, including long-term studies. While newer ALK inhibitors are associated with fewer AES, crizotinib, ceritinib and alectinib all represent valuable therapeutic options. Management of AEs will improve the therapeutic index of these agents and enhance quality of life for patients.













