In 2015, the prevalence of patients living with lung cancer in the US is more than 430,000 and the incidence of new cases of lung cancer is around 221,200.1 Deaths from lung cancer are estimated to be 158,040.1 In Europe, in 2012, 410,000 patients were diagnosed with lung cancer and 354,000 died from the disease.2,3 Data from the National Lung Cancer Audit (LUCADA) for England in 2011 show that the majority of lung cancers (87 %) are classified as non small cell lung cancer (NSCLC).4
Treatment decisions for patients with adenocarcinoma, which account for around 40 % of lung cancers, are increasingly being made based on driver mutations and other genomic abnormalities (i.e. anaplastic lymphoma kinase [ALK] and ROS1 translocations). Driver mutations are causally implicated in oncogenesis, confer a growth advantage to cancer cells, and are positively selected in the microenvironment of the cancer tissue.5 Tumors from patients with advanced NSCLC are screened routinely for mutations in the kinase domain of the epidermal growth factor receptor (EGFR). Somatic EGFR mutations occur in approximately 10–30 % of patients with NSCLC6 and are around twice as common in East Asian patients compared with Western patients.7 The majority (approximately 90 %) of EGFR-mutation-positive patients have one of two types of
activating mutations at the time of diagnosis – a deletion in exon 19 or an L858R point mutation in exon 21.7
The first- and second-generation EGFR tyrosine kinase inhibitors (TKIs), gefitinib,8 erlotinib,9 and afatinib10 are effective against lung cancers with these mutations; however, acquired resistance develops within a median of 9 to 13 months, limiting their duration of efficacy. Acquired resistance of NSCLC to EGFR TKIs develops through various molecular mechanisms, principally T790M secondary mutation, but also MET amplification, hepatocyte growth factor (HGF) overexpression, PTEN downregulation, and epithelial-mesenchymal transition (EMT) among others.11 Most commonly, however, this resistance is due to the EGFR T790M mutation (the “gatekeeper” residue), which is present in around 50–60 % of resistant cases.12,13 This mutation occurs in the adenosine triphosphate (ATP) binding site of the kinase14 and leads to a raised affinity for ATP, thereby reducing the ability of ATP-competitive reversible EGFR tyrosine TKIs to bind to the tyrosine kinase domain of EGFR.15 Wild-type EGFR inhibition can lead to cutaneous toxicity and diarrhoea.16 The median survival after the emergence of the T790M mutation is under 2 years and there are, as yet, no approved therapies that specifically target this mutation. Therefore, cytotoxic chemotherapy is the current standard of care for such patients.
Further, the T790M gatekeeper mutation has been detected in some patients prior to drug exposure.17,18 The frequency of a pre-existing sporadic EGFR T790M mutation has varied widely in the literature: from 2 % to 60 %.13,19–25 In the largest series reporting mechanisms of acquired resistance to EGFRTKI therapy, 98/155 (63 %) had second-site EGFR T790M mutations.13 The median time to develop drug resistance to EGFR-TKIs has been estimated to be between 6 to 12 months.26 Median survival is in the region of <2 years after the emergence of T790M.13 The presence of this mutation before treatment reduces the efficacy of first-generation EGFR-TKIs in patients with EGFR-mutant lung cancer.22,24,25 In this patient population, therefore, it will be beneficial to be able to detect resistant mutations prior to making treatment decisions to enhance personalized therapy.
Two EGFR TKIs, rociletinib (CO-1686; Clovis Oncology, CO, USA) and AZD9291 (AstraZeneca, London, UK), have been shown in preclinical studies to be effective against T790M resistance mutations.27,28 Alternative treatments for NSCLC that may be capable of overcoming EGFR T790M mutant-based TKI Resistance include allele-specific DNAzyme, such as DzT, and T790M-specific-siRNAs.29,30
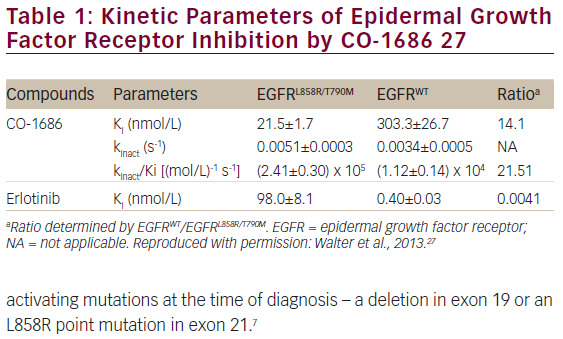
Rociletinib is an oral, irreversible, potent, covalent inhibitor of the activating EGFR mutations (del19 and L858R) and the T790M resistance mutation, with sparing of wild-type EGFR (see Figure 1 and Table 1).27 In xenograft models
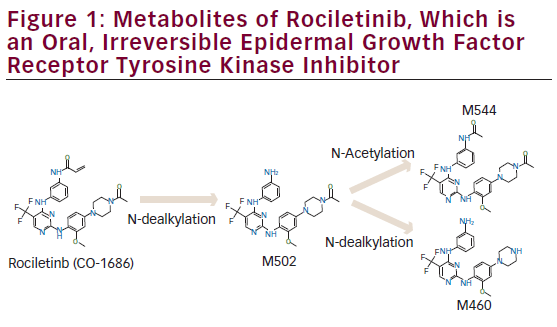
with EGFR-activating mutations or T790M resistance mutations, rociletinib led to durable tumor shrinkage, especially when plasma concentrations of rociletinib were maintained at more than 200 ng/mL across the doses tested.27 Given these promising results, a phase I–II (TIGER-X) study was performed. Rociletinib, which has an elimination half-life of 2–4 hours, is also being investigated in single-arm studies in mutant EGFR NSCLC patients with acquired resistance to TKIs and in TIGER-1 and TIGER-3 in comparative studies. Rociletinib received Breakthrough Therapy designation from the US Food and Drug Administration (FDA) in May 2014.
The TIGER Trials—A Clinical Development Program for Rociletinib in Patients with Mutant Epidermal Growth Factor Receptor Non-small Cell Lung Cancer
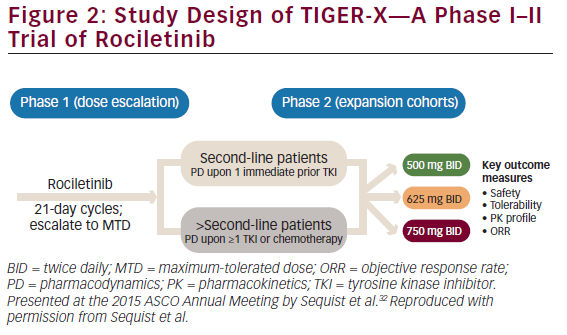
TIGER-X—Phase I–II
TIGER-X (NCT01526928, Figure 2) is evaluating rociletinib in two groups of patients:31,32
• patients who have progressed following their first and only EGFRdirected TKI therapy who have developed the T790M mutation; and
• later-line T790M positive patients who have progressed on their second or later TKI therapy or subsequent chemotherapy.
Additional eligibility criteria include: age ≥18 years, an Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a scale of 0–5 with 0 indicating no symptoms), and adequate organ function. All patients needed to undergo tumor biopsy during screening for assessment of EGFR mutation status. There is no contraindication for pre-existing diabetic patients.
The study consists of two parts, starting with a phase I dose-escalation study. In the phase II part of the study, patients with T790M-positive NSCLC received rociletinib 500 mg twice daily (BID), 625 mg BID, or 750 mg BID. In phase I, patients are not restricted to those with T790M-positive status although in phase II, central confirmation of T790M-positive status was required. The main objectives of TIGER-X are to study the safety and tolerability, pharmacokinetics, and the preliminary anti-tumor activity of rociletinib.
Patient Characteristics/
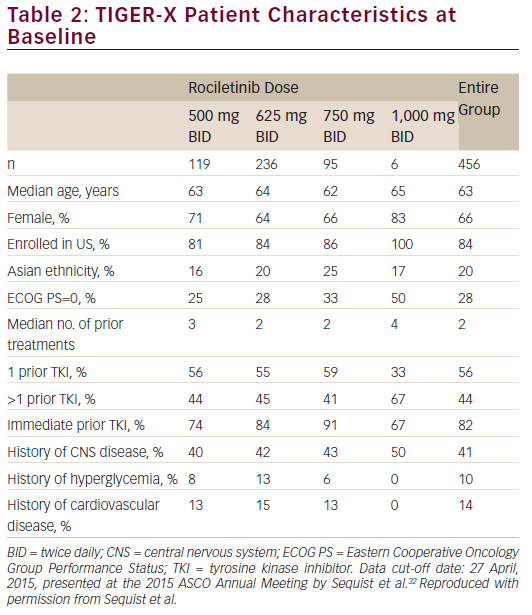
As of April 27, 2015, 456 patients (119 of whom received rociletinib 500 mg BID) were enrolled at 10 centres across the United States, France, and Australia (see Table 2). The majority of patients were female (66 %) and were enrolled in the US (84 %). Only 20 % of patients were of East Asian ethnicity; approximately 80 % were Western patients. Approximately 10 % of patients had a history of diabetes or glucose impairment. Overall, the median number of prior treatments was three for those on the 500 mg BID dose, and 82 % of patients had a TKI as their immediate prior line of therapy. In addition, 41 % of patients had a history of central nervous system (CNS) disease.
Efficacy
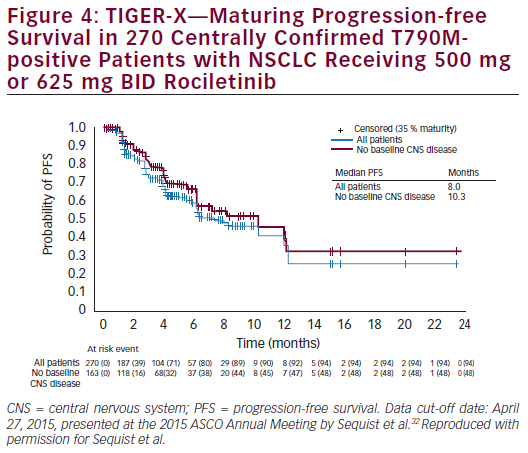
Across all doses (500–1,000 mg BID), patients with centrally confirmed T790M positive disease had an objective response rate (ORR) of 53 % and a disease control rate (DCR) of 85 % (see Figure 3). Patients enrolled at the 500 mg BID dosing level had an ORR of 60 % and a DCR of 90 %. The efficacy in terms of response did not improve with increasing dose. The median progression free survival (PFS) for all patients was 8 months. In patients with no baseline CNS disease, median PFS was 10.3 months (see Figure 4). The ORR was 35–45 % among T790-negative patients.
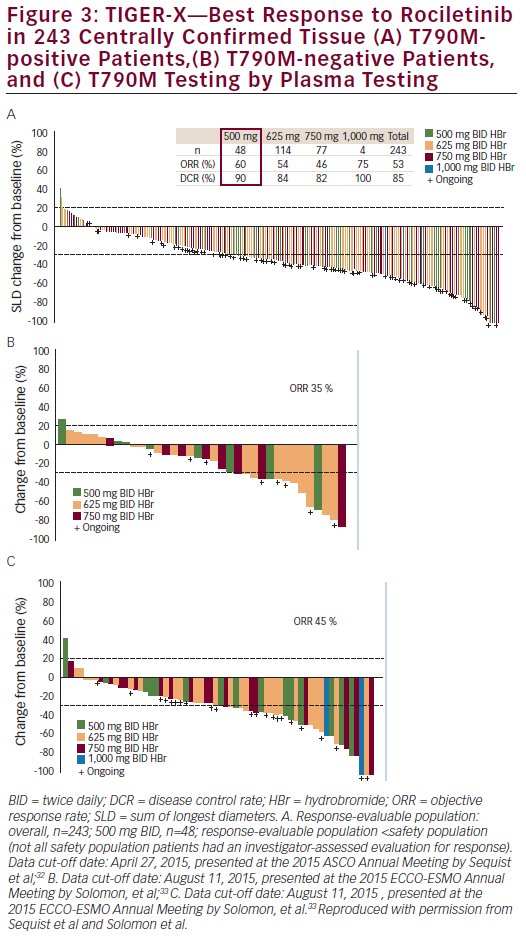
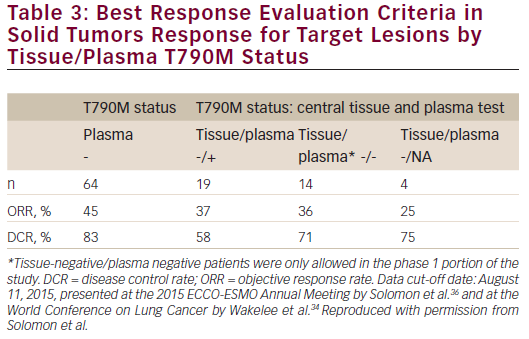
At the 2015 ECC-ESMO Annual Meeting in Vienna, Austria, updated results in centrally confirmed tissue T790M-negative patients were presented (data cut-off date: August 11, 2015; n=43 for safety analysis; n=37 for efficacy evaluable analysis). ORR and DCR based on tissue testing for T790M-negative status using central results (n=37) were 35 % and 65 %, respectively. Response rates for patients with mixed or missing tissue/plasma T790M mutation status are shown in Table 3.33,34 Response rates in patients with centrally confirmed T790M-negative disease and T790M-negative disease by plasma testing were 35 % and 45 %, respectively.
Safety
Common treatment-related adverse events with rociletinib are listed in Table 4. Acneiform rash was not observed, although one patient had a grade 1 maculopapular rash.31 No interstitial lung disease was observed in the 500 mg BID dose group; there were 7/456 cases (1.5 %) overall. There
was also no paronychia or stomatitis. Treatment-related adverse events leading to drug discontinuation were seen in only 2.5 % of patients in the 500 mg BID dosing cohort (4 % overall).
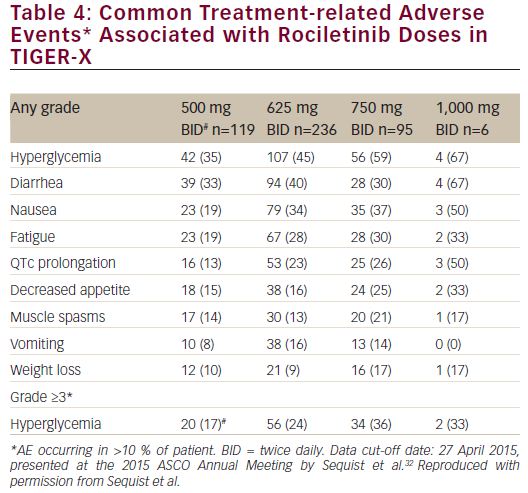
Across all dosing levels, a dose-dependent relationship was apparent between rociletinib treatment and hyperglycemia and QTc prolongation.35 Hyperglycemia is believed to be caused by insulin resistance mediated by a rociletinib metabolite (M502) that inhibits IGF1R/IR. This adverse event was not expected in humans since it was not observed in preclinical studies. Once hyperglycemia was identified, monitoring and treatment guidelines (e.g., monitor blood and/or urine glucose; treat when necessary with oral anti-hyperglycemic agents) was introduced into trial protocols; this approach has been successful in reducing grade ≥3 hyperglycemia in the TIGER-X study. In the 500 mg BID group, nine of 94 patients (9.6 %) without a history of diabetes or glucose impairment had post-baseline glucose measurements that exceeded 250 mg/dL at least two times. Across all three dosing groups (500 mg BID, 625 mg BID, and 750 mg BID), 29 of 382 patients (7.6 %) without a history of diabetes or glucose impairment had post-baseline glucose measurements that exceeded 250 mg/dL at least twice.
Conclusions
In the ongoing TIGER-X study, rociletinib has shown activity in patients with EGFR mutant NSCLC patients associated with the T790M mutation and is well tolerated. Promising activity has also been observed in patients with T790M-negative disease. An ORR of 60 % and DCR of 90 % in centrally confirmed tissue T790M-positive patients were recorded at the 500 mg BID dosing level. The discontinuation rate due to adverse events was low at 2.5 %. The current immature estimates of overall median PFS is 8.0 months in all centrally confirmed tissue T790M-positive patients and 10.3 months in patients without baseline CNS disease. The median number of prior treatments was three for those on the 500 mg BID dose and 82 % of patients had a TKI as their immediate prior line of therapy, which decreases the likelihood or potential of activity being a reflection of an EGFR retreatment effect. ORRs were encouraging among patients with T790M-negative NSCLC; ongoing trials continue
to study patients with T790M-negative disease. Possible reasons why we see T790M-negative activity include: tumor heterogeneity,36 IGF1R signaling, assay sensitivity, and the importance of targeting activating EGFR mutations (del19 and L858R) as opposed to resistance mutation (T790M). TKI retreatment effect is not a likely explanation because the majority of patients had a recent history of progression on an EGFR TKI; immediate EGFR TKI use was also 82 % in all dosing cohorts, ruling out a retreatment effect.
In TIGER-X, 41 % of patients had a history of CNS disease, which is associated with significant morbidity and mortality.37 Further, only 20 % of the population was of East Asian ethnicity, which may be clinically meaningful because there is evidence from clinical trials that East Asian ethnicity may be a prognostic indicator of response to treatment with EGFR TKIs.9,38 In the OPTIMAL study (ClinicalTrials.gov, number NCT00874419), which compared erlotinib with chemotherapy as first-line treatment for patients with advanced EGFR mutationpositive non-small-cell lung cancer, 100 % of the study population were of Asian ethnicity. The ORR (Response Evaluation Criteria in Solid Tumors [RECIST]) was 82 % and median PFS was 13.1 months.38 This contrasts with the results of the erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC) study (ClinicalTrials.gov, number NCT00446225), in which 98 % of the population were of Western ethnicity, who, therefore, may be expected to respond to treatment less successfully than the Asian population in the OPTIMAL study.9 The ORR (RECIST) was 58 % and the median PFS was 9.7 months.
Ongoing TIGER Programme
In addition to the TIGER-X study, rociletinib is being investigated in other lines of therapy.
TIGER-2
(ClinicalTrials.gov, number NCT02147990) is an ongoing phase II, open-label, multicentre, safety and efficacy study of oral rociletinib as second-line EGFR-directed TKI in patients with mutant EGFR NSCLC (see Figure 5). Patients are currently being enrolled; the estimated study completion date is May 2017. The primary objective of TIGER-2 is to evaluate the anti-tumor efficacy of single-agent rociletinib as measured by ORR, when administered to patients with EGFR-mutated, centrally
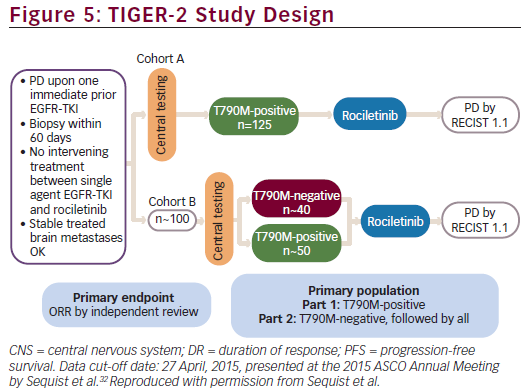
confirmed T790M-positive and T790M-negative advanced NSCLC following tumor progression on previous EGFR-directed TKI. Secondary objectives include assessment of efficacy by DCR, duration of response (DR), PFS, and overall survival (OS), and assessments of pharmacokinetic (PK) profile, safety, and tolerability. Key inclusion criteria include: histologically or cytologically confirmed metastatic or unresectable, locally advanced NSCLC; evidence of a tumor with one or more EGFR mutations; disease progression confirmed while receiving a first-line single agent EGFR-TKI; central laboratory confirmation of the presence of the T790M mutation in tumor tissue in Cohort A and the presence or absence of the T790M mutation in tumor tissue in Cohort B; age ≥18 years; and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Main exclusion criteria are: an exon 20 insertion activating mutation in the EGFR gene; active second malignancy; i.e. patient known to have potentially fatal cancer present for which he/she may be (but not necessarily) currently receiving treatment; and known pre-existing interstitial lung disease.
TIGER-3
The aim of TIGER-3 (NCT02322281) is to compare the anti-tumor efficacy of rociletinib with that of single-agent cytotoxic chemotherapy in patients with EGFR-mutated, advanced/metastatic NSCLC after failure of at least one previous EGFR-directed TKI and at least one line of platinumcontaining doublet chemotherapy. In TIGER-3, single-agent cytotoxic chemotherapy was the investigator’s choice of pemetrexed, gemcitabine, docetaxel, or paclitaxel; choice of chemotherapy agent was to be specified before randomization.
The primary outcome measure in this study is PFS whereas secondary outcome measures include ORR, duration of response (DOR), DCR, OS, plasma PK parameters, and safety. Key inclusion criteria include: disease progression confirmed by radiological assessment while receiving treatment with single-agent EGFR TKI (eg., erlotinib, gefitinib, afatinib, or dacomitinib); biopsy of either primary or metastatic tumor tissue within 60 days prior to start of treatment for central biomarker testing (results not required for enrolment); and evidence of a tumor with ≥1 EGFRactivating mutations excluding exon 20 insertion. Key exclusion criteria are: any other malignancy associated with a high mortality risk within the next 5 years; known pre-existing interstitial lung disease; and tumor small cell transformation by local assessment, irrespective of the presence of T790M-positive component.
TIGER-1—Phase II/III
The aim of TIGER-1 (NCT02186301) is to compare the safety and antitumor acitivity of rociletinib with erlotinib in patients whose tumors have specific EGFR mutations and who have not previously received any EGFR TKI therapy. The primary outcome measure is PFS whereas the secondary outcome measures include: DCR, safety, plasma PK parameters, OS, ORR, and DOR. The key inclusion criteria are: patients with histologically or cytologically confirmed metastatic or unresectable locally advanced recurrent NSCLC; documentation of ≥1 activating EGFR mutation; and undergone a biopsy or surgical resection of either primary or metastatic tumor tissue within 60 days. Key exclusion criteria are: exon 20 insertion activating mutation in the EGFR gene; and prior chemotherapy in the metastatic setting; and CNS disease. Enrolment of the phase II portion of the study is expected to be completed in June 2016.
New Directions for the TIGER Programme
The TIGER programme is ongoing and a future aim is to investigate whether rociletinib treatment leads to an improvement in OS. In August 2015, a New Drug Application (NDA) was submitted to the US FDA for rociletinib for the treatment of patients with mutant EGFR NSCLC who have previously been treated with an EGFR-targeted therapy and who carry the EGFR T790M mutation.39 A Marketing Authorization Application (MAA) has also been submitted to the European Medicines Agency (EMA) through the centralized procedure for rociletinib for the treatment of adult patients with mutant EGFR NSCLC who have been previously treated with an EGFRtargeted therapy and have the EGFR T790M mutation.
Ultimately, rociletinib may be partnered with other anti-cancer agents such as anti-programmed cell death protein and programmed cell death 1 ligand 1 monoclonal antibodies, mitogen-activated protein kinase enzyme inhibitors, vascular endothelial growth factor inhibitors, and c-Met inhibitors. Recent analysis of acquired rociletinib resistance (n=20)has not identified a consistent mechanism of resistance.32 Following further investigation, it may become possible to delay the emergence of a new rociletinib resistance mutation by pairing rociletinib with another agent that targets that specific resistance mechanism. For example, MET amplification is known to occur following use of rociletinib in a minority of cases; therefore, a combination of rociletinib with crizotinib, which targets tumor cells with MET amplification, may be more effective than rociletinib alone.
Other future directions include the investigation of sequencing, combination therapies, and understanding emerging data on mechanisms of resistance, including C797S and MET amplification.32













