Lung cancer is the most common cancer in the US with an estimated 221,200 new cases in 2015. An estimated 158,040 patients will die of the disease this year.1 The overall 5-year survival rate for all stages of lung cancer is 17 %, and for advanced disease, 4 %.1 Lung cancer will account for approximately 27 % of all cancer deaths in the US in 20151; these statistics underscore the need for effective therapeutic agents in this patient population.
Histologically, the majority (85 %) of lung cancers are classified as non-small cell lung cancers (NSCLCs),2 comprising squamous cell carcinoma (25–30 % of all lung cancers), adenocarcinoma (~40 %), and large-cell carcinoma (10– 15 %).3 Advances in the past decade now allow for treatment of NSCLC based on molecular subtype versus solely by histologic subtype of the tumor.2,4 Genetic alterations that contribute to the pathogenesis of NSCLC and are reasonable targets for pharmacotherapies have been identified, including epidermal growth factor receptor (EGFR)-activating gene mutations and anaplastic lymphoma kinase (ALK) gene rearrangements.5–8 Other emerging targets involving genetic alteration include ROS1 rearrangements, BRAF V600E mutation, mesenchymal epithelial transition factor (MET) amplification, human epidermal growth factor 2 (HER2) mutations, and rearranged during transfection (RET) rearrangements.2,9
The prevalence of EGFR mutations (EGFRm) in NSCLC ranges from 7–14 % in Western populations and 30–50 % in Asian populations.2,7,10–13 Among patients with NSCLC, EGFRm is more commonly seen in women (42 %) versus men (14 %); in never smokers (51 %) versus smokers (10 % ); and in adenocarcinoma histology (40 %) versus nonadenocarcinomas (3 %).11
EGFRm drive tumor growth and progression by activating cell survival and proliferation signal transduction cascades.14 A subset of these activating EGFRm are commonly referred to as sensitizing mutations because their presence is predictive of response to EGFR tyrosine kinase inhibitors (TKIs).5,6 The most common sensitizing EGFR gene mutations are deletions in exon 19 (ex19del; 45–49 %) and point mutations in exon 21 (L858R; 40 %).2,11,15–18
Similarly, ALK-rearranged patients treated with ALK inhibitors such as crizotinib have demonstrated high overall response rates.19 Therefore, guidelines from the National Comprehensive Cancer Network (NCCN), National Cancer Center, American Society of Clinical Oncology, College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology all recommend routine EGFR and ALK testing and strongly recommend molecular profiling to identify rare actionable alterations that already have effective agents or are being studied in clinical trials for patients with nonsquamous adenocarcinoma NSCLC.2,4,20
This review examines the clinical evidence supporting three EGFR TKIs currently available for first-line treatment of EGFRm-positive metastatic NSCLC in the US. It also discusses resistance mechanisms acquired by tumors after treatment with EGFR TKIs and how to identify the actionable mutations at disease progression. Lastly, current and future therapies for treatment of EGFR TKI-resistant NSCLC are reviewed.
EGFR Tyrosine Kinase Inhibitors
The current NCCN guidelines treatment paradigm recommends EGFR inhibitors as a first-line treatment for patients with EGFRm-positive advanced or metastatic NSCLC.2 The available TKIs for first-line treatment of metastatic NSCLC are gefitinib (IRESSA®; AstraZeneca, Wilmington, DE), erlotinib (Tarceva®; OSI Pharmaceuticals, LLC, Farmingdale, NY), and afatinib (GILOTRIF®; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT) with response rates ranging from 56 to 84 % and a median progressionfree survival (PFS) in the range of 8–14 months.12,13,16,17,21–25
Gefitinib
Gefitinib is a first-in-class EGFR TKI that was initially introduced in 2002 in Japan and other parts of the world.26,27 Gefitinib was introduced to the US market in 2003; however, in 2005 its use was restricted based on lack of survival benefit in an unselected patient population in a phase III trial.26 Following the discovery of EGFRm,5,6 gefitinib became available in the EU in 2009.27 More recently, gefitinib was reintroduced in the US for the firstline treatment of EGFRm-positive metastatic NSCLC, based on the results of two clinical trials.28 IRESSA® Follow-Up Measure (IFUM) was an openlabel, single-arm, phase IV, bridging study of first-line gefitinib conducted in Caucasian patients with EGFRm-positive advanced NSCLC (aNSCLC; n=106). Gefitinib-treated patients experienced an objective response rate (ORR) of 70 % (95 % confidence interval [CI], 61–78), median PFS of 9.7 months, and median overall survival (OS) of 19 months.12 Supporting these results was a subset analysis of the Iressa Pan-Asia Study (IPASS). The IPASS trial was a phase III, randomized, open-label, multicenter, parallelgroup study in an Asian population that compared first-line gefitinib with carboplatin + paclitaxel chemotherapy in a subset of patients with EGFRm-positive aNSCLC (n=186).17,28 For patients with EGFRm-positive tumors the median PFS (as assessed by blinded independent central review) was 10.9 months for gefitinib compared with 7.4 months for chemotherapy (hazard ratio [HR] 0.54; 95 % CI 0.38–0.79).28 Although median OS did not differ between treatment groups (gefitinib, 21.6 months versus chemotherapy, 21.9 months; HR 1.0; 95 % CI 0.76–1.33),21 the ORR was greater for gefitinib-treated patients (67 %; 95 % CI 56–77 %) compared with patients treated with chemotherapy (41 %; 95 % CI 31– 51 %).28 The ORR and PFS in this Asian patient population were similar to those observed in the IFUM Caucasian population17,12 and confirm the consistent efficacy of gefitinib in patients of different ethnicities with EGFRm-positive aNSCLC.
Erlotinib
Erlotinib was approved in the US in 2004 for treatment of locally advanced or metastatic NSCLC after failure of at least one prior chemotherapy regimen.29 It was subsequently approved in 2010 as maintenance treatment for patients without disease progression after four cycles of platinum-based, first-line chemotherapy.29 After the discovery of EGFRm, erlotinib received approval in 2013 as first-line treatment in patients with EGFRm-positive metastatic NSCLC, based on the results of an open-label, randomized, phase III study (the European Tarceva versus Chemotherapy [EURTAC] trial).13 Caucasian patients with EGFRm-positive aNSCLC treated with erlotinib experienced significant improvement in median PFS (10.4 months) compared with those who received a standard-of-care platinumbased doublet chemotherapy (5.2 months; HR 0.34; 95 % CI 0.23–0.49; p<0.0001).29 Whereas there was no difference in median OS between erlotinib-treated and chemotherapy-treated patients (22.9 months versus 19.5 months, respectively; 95 % CI 0.64–1.35; p=0.93) the ORR was higher for erlotinib versus chemotherapy (65 %; 95 % CI 54.1–75.1 % versus 16 %; 95 % CI 9.0–25.3 %, respectively).29 Although not included in the US label, the OPTIMAL (CTONG-0802) trial was an open-label, randomized, phase III trial of first-line erlotinib versus chemotherapy in Asian patients with EGFRmpositive aNSCLC.23 The median PFS was 13.1 months (95 % CI 10.58–16.53) for erlotinib-treated patients compared with 4.6 months (95 % CI 4.21– 5.42) for those treated with chemotherapy (HR 0.16; 95 % CI 0.10–0.26; p<0.0001).23 Similar to gefitinib, the erlotinib trials demonstrated efficacy in both Asian and Caucasian patients with EGFRm-positive aNSCLC.
Afatinib
Afatinib was approved in the US in 2013 as first-line treatment of EGFRmpositive metastatic NSCLC, based on the results of the LUX-Lung 3 trial.16,30 Patients with EGFRm-positive aNSCLC who were treated with afatinib experienced PFS of 11.1 months compared with 6.9 months in patients treated with pemetrexed/cisplatin (HR 0.58; 95 % CI 0.43–0.78; p<0.001), with an ORR of 50.4 % versus 19.1 %, respectively.16,30
Similar to the results seen with other EGFR TKIs, data from both the LUX-Lung 3 and LUX-Lung 6 (a second open-label, randomized, phase III trial) demonstrated no difference in OS with afatinib compared with chemotherapy (LUX-Lung 3: HR 0.88; 95 % CI 0.66–1.17; p=0.39; LUX-Lung 6: HR 0.93; 95 % CI 0.72–1.22; p=0.61).31 However, a preplanned analysis of these two trials revealed a significant improvement in OS with afatinib compared with chemotherapy for patients with an EGFR ex19del mutation (LUX-Lung 3: HR 0.54; 95 % CI 0.36–0.79, p=0.0015; LUX-Lung 6: HR 0.64; 95 % CI 0.44–0.94; p=0.023), but not for patients with the L858R mutation (LUX-Lung 3: HR 1.3; 95 % CI 0.80–2.11; p=0.29; LUX-Lung 6: HR 1.22; 95 % CI 0.81–1.83; p=0.34).31 The reason for such a difference by EGFRm subtype is not fully understood. However, other studies, including a recent meta-analysis, support improved outcomes in patients treated with gefitinib, erlotinib, or afatinib who harbor an EGFR ex19del mutation compared with L858R mutation-positive tumors.32–34 Data from other EGFR TKI studies support these observations. In the EURTAC trial, patients in the ex19del subgroup treated with erlotinib had significant improvements in median PFS compared with chemotherapy (HR 0.30; 95 % CI 0.18–0.50; p<0.0001) but improvement in PFS for patients with L858R mutations was
not statistically significant (HR 0.55; 95 % CI 0.29–1.02; p=0.0539).13 In the IPASS trial, when compared with chemotherapy, gefitinib-treated patients in both mutational subgroups had statistically significant improvements in median PFS (ex19del: HR 0.38; 95 % CI 0.26–0.56; L858R: HR 0.55; 95 % CI 0.35–0.87); however, the ex19del subgroup had a slightly greater advantage.21 These data suggest different EGFR gene mutations may carry different prognoses.34
EGFR Tyrosine Kinase Inhibitor Safety and Tolerability Profiles
A recent meta-analysis of 28 studies of gefitinib, erlotinib, and afatinib in patients with aNSCLC demonstrated an overall similarity in efficacy among the three agents.35 Their safety and tolerability profiles differ, however, and afatinib may be associated with a worse tolerability profile.35 Clinical differences between TKIs may be explained by the molecular differences in mechanism of action, structure, and affinity for wild-type (wt) EGFR.36,37 Gefitinib and erlotinib are reversible EGFR TKIs that block EGFR activation by binding to the adenosine 5’-triphosphate (ATP) binding site.36,38 These agents have increased binding affinity for mutant EGFR compared with wtEGFR.36 Afatinib is an irreversible EGFR TKI that covalently binds to mutant EGFR, wtEGFR, and other members of the ErbB family, HER2 and HER4.37
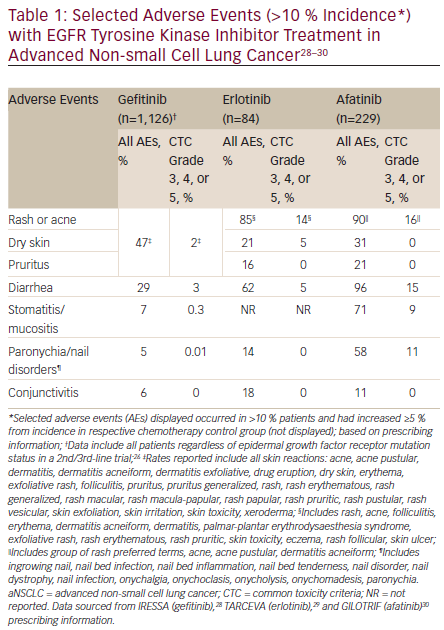
Selected adverse events (AEs) reported in the prescribing information for each agent are provided in Table 1. Incidence of all grades of skin reactions (including rash) were lower with gefitinib compared with afatinib and erlotinib. Similarly, there were lower rates of all grades of diarrhea with gefitinib compared with the other two agents, while both gefitinib and erlotinib reported lower rates of ≥ grade 3 diarrhea compared with afatinib. Afatinib also had higher rates of all grades of stomatitis/mucositis and paronychia compared with erlotinib and gefitinib. The reported discontinuation and dose reduction rates due to AEs for these agents markedly differ.28–30 Discontinuation rates due to AEs were 14.3 % for patients treated with erlotinib, 14 % for afatinib, and 3 % for gefitinib. Dose interruptions or reductions due to AEs occurred in 37 % of erlotinib-treated and 57 % of afatinib-treated patients. Dose reductions due to AEs were not reported in the gefitinib prescribing information.28–30 Evaluation of each agent’s safety and tolerability profile is particularly important to treatment selection, as there are no data available to distinguish between the three first-line therapeutic options in metastatic NSCLC. Additionally, patients receiving EGFR TKIs should be monitored to assess treatment response, tolerability to therapy, disease progression, and potential acquired resistance mechanisms.2,39
EGFR Tyrosine Kinase Inhibitor Resistance Mechanisms
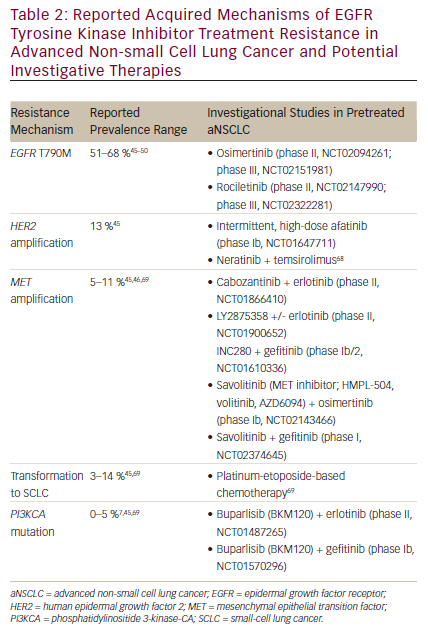
Inevitably, acquired resistance to first-line EGFR TKIs occurs in most patients with a median PFS of approximately 12 months (range, 8–14 months) after treatment initiation.12,13,16,17,21–25 Clinical progression after EGFR TKI therapy can be broadly categorized into three subtypes, each requiring a different treatment approach.40 The first subtype is oligoprogressive disease, defined as progression at new sites or a limited number of areas with regrowth. The second, central nervous system (CNS) sanctuary progressive disease, refers to isolated CNS failure (e.g., brain metastasis) without systemic progression. In this case, progression in the CNS may be the result of poor penetration of the blood–brain barrier by TKIs.40 For oligo-progressive disease and CNS sanctuary progressive disease, it is reasonable to institute local therapy with radiation while continuing TKI therapy.2,40 The last subtype, systemic progressive disease, is defined as multisite progression with possible new metastatic sites and regrowth in previously responsive areas.40 There are multiple approaches to treating systemic progressive disease after EGFR TKI resistance, including continuing on the same TKI, switching to chemotherapy, or adding on a therapy—such as chemotherapy or another targeted agent—to the TKI regimen.2,40 It is important to note that these approaches have limited efficacy in progressive disease after EGFR TKI therapy. In the ASPIRATION study, continuation with erlotinib therapy post progression demonstrated a median PFS of only 3.7 months.41 Furthermore, the recent IMPRESS trial demonstrated no statistically significant PFS improvement by continuing gefitinib with the chemotherapy after progression on gefitinib.42,43 The molecular mechanisms that generate EGFR TKI resistance are many and are distinct from the original tumor, thus they need to be managed differently.44 Among them, the most common resistance mechanism is the gatekeeper mutation in the EGFR kinase domain (EGFR T790M), followed by HER2 gene amplification, MET gene amplification, transformation to small-cell carcinoma, and phosphatidylinositide 3-kinase-CA (PIK3A) gene mutation.44,45 As more mechanisms of resistance are discovered it becomes increasingly important to not only identify them at disease progression, but also to understand which of these mechanisms can be targeted with currently available agents (see Table 2).
EGFR T790M
A substitution of methionine for threonine at the 790 position of exon 20 (T790M) in the EGFR kinase domain is the most common EGFRm, with a reported incidence ranging from 51 to 68 % in patients whose disease has progressed on erlotinib and gefitinib.45–50 As afatinib is the newest of the TKIs, there are limited data reporting the incidence of T790M resistance with afatinib disease progression. However, an in vitro model of acquired resistance to first-line afatinib demonstrated that T790M mutations may occur.51 Though afatinib appears to have some activity in patients who progressed on other EGFR TKIs (LUX-Lung 1 study),52 a more recent study showed no response in patients with a T790M-acquired mutation.53 In a phase Ib trial, the combination of afatinib plus cetuximab in pretreated patients with EGFRm-positive acquired resistance to gefitinib/erlotinib demonstrated a median PFS of 4.7 months (95 % CI 4.3–6.4).54 However, larger-scale studies are needed to determine its efficacy and toxicities in comparison with EGFR TKIs that specifically target T790M. Perhaps the combination may be useful in resistant tumors that do not harbor T790M.
The T790M mutation is thought to be resistant to EGFR TKIs through several mechanisms, including steric hindrance from the introduction of a bulkier amino acid (reduced binding of reversible TKIs), increased binding affinity of ATP, and increased phosphorylation levels, leading to reduced potency of TKIs.55–57 Multiple novel agents are in clinical development that target the T790M mutation, including osimertinib (AZD9291; AstraZeneca, Wilmington, DE), ASP8273 (Astellas Pharma US, Inc., Northbrook, IL), rociletinib (CO-1686; Clovis Oncology, San Francisco, CA), and HM61713 (Hanmi Pharmaceutical Co, Seoul, Korea). Osimertinib and rociletinib are in phase II clinical development and are discussed in greater detail here.
Osimertinib is an oral, once-daily, irreversible EGFR TKI specifically designed to target both the T790M mutation and mutant EGFR with low activity against wtEGFR, the insulin receptor (IR), and the insulinlike growth factor receptor type 1 (IGF-1R).58–60 An interim analysis of a phase I/II study demonstrated that patients with T790M mutation-positive aNSCLC (n=59) in the osimertinib 80 mg once-daily arm experienced an ORR of 54 % (95 % CI 41–67 %) and a duration of response of 12.4 months (95 % CI 8.3–not calculable) with a median PFS of 13.5 months (95 % CI 8.3–not calculable; 38 % data maturity by central reviewers).61 In this same cohort, investigator-assessed data demonstrated an ORR of 66 % (95 % CI 52–77 %) with the duration of response not calculable (95 % CI 8.2–not calculable) and a median PFS of 10.9 months (95 % CI 8.3–not calculable; 40 % data maturity). AEs across all 103 osimertinib-treated patients were mostly mild (grade 1/2) with the most commonly reported being rash (38 %) and diarrhea (36 %).61 In this safety population, rates of hyperglycemia were low (4 %),61 likely because osimertinib was designed to have minimal activity against IR and IGF-1R.58 Osimertinib is currently under investigation as a first-line therapy verses standard of care in a phase III study (NCT02296125).62
Rociletinib (CO-1686) is an oral, twice-daily, irreversible EGFR TKI that targets both the T790M mutation and mutant EGFR with low activity against wtEGFR. Additionally, a metabolite of rociletinib is reported to be an inhibitor of IGF-1R.63,64 An updated interim analysis of a phase I/II study was recently presented and showed an ORR of 60 % and a disease control rate of 90 % in T790M mutation-positive aNSCLC patients who received the recommended 500 mg dose (n=48).65 Median PFS was
8.0 months (35 % data maturity) in T790M mutation-positive patients treated with 500 g or 625 mg of rociletinib (n=270). Safety analysis for the 119 patients treated with all formulations of rociletinib 500 mg reported that treatment-related AEs were generally mild. Although there were no reports of rash, diarrhea was reported in 33 % of patients. Hyperglycemia was reported in 35 % of patients, of which 17 % were classified as grade 3/4.65 The hyperglycemia associated with rociletinib has been linked to the inhibition of the IGF-1R.63,64
In 2014, the US Food and Drug Administration granted breakthrough therapy designation for rociletinib and osimertinib. Both therapies appear to have similar efficacy; however, their tolerability profiles differ, possibly reflecting their different affinities for wtEGFR and other receptors such as IGF-1R. Updated data from the ongoing clinical trials for both therapies are expected. The approval of these agents in the US is eagerly anticipated and will provide a much needed option for patients with T790M mutationpositive aNSCLC.
HER2 Amplification
HER2 amplification occurs in approximately 13 % of patients with aNSCLC and acquired resistance to EGFR TKIs.45 HER2 is a tyrosine kinase within the same family as EGFR; and amplification of the HER2 gene may
mediate EGFR TKI resistance by stimulating the same downstream cell growth and survival pathways, such as the phosphatidylinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway.66
There are a few agents under investigation that target HER2 in pretreated aNSCLC (see Table 2). Dacomitinib, an irreversible pan-HER inhibitor, was investigated in a double-blind, randomized, phase III trial in heavily pretreated patients with aNSCLC and did not demonstrate a significant increase in OS compared with placebo.67 Intermittent, high-dose afatinib,44 as well as combination therapy with neratinib plus temsirolimus,68 show promising results in aNSCLC in phase I trials.
MET Amplification
The reported prevalence of secondary MET amplification in aNSCLC is between 5–11 %.45,46,69 MET activates the PI3K pathway, which bypasses the need for EGFR activation.44 Combining an agent that blocks the MET pathway while inhibiting EGFR may prevent the resistance/bypass mechanism from being selected.70 This was investigated in a phase II trial of onartuzumab plus erlotinib in patients with recurrent aNSCLC. The combination showed significant improvement in PFS (HR 0.53; p=0.04) and OS (HR 0.37; p=0.002) in MET-positive patients treated with the combination therapy versus erlotinib alone (n=66)71; however, a subsequent phase III trial using the same combination showed no OS advantage,72 perhaps due to having not selected EGFRm-positive patients.73 Exploratory analyses are now underway for different molecular subgroups.72 Other MET inhibitor combinations currently under investigation in EGFR TKI-resistant aNSCLC are listed in Table 2.
Transformation to Small-cell Lung Cancer
Two studies found that histologic transformation of aNSCLC to small-cell lung cancer (SCLC) occurs in approximately 3–14 % of patients with EGFR TKI-acquired resistance.45,69 An alternative theory suggests that this may not be transformation, but rather that an SCLC clone is associated with NSCLC and as the adenocarcinoma is treated with TKI therapy, the SCLC is able to grow.74 In Sequist et al., four patients who had SCLC were treated with the classic SCLC treatment, platinum-etoposide–based chemotherapy, and a 60 % response rate was observed.69
PI3KCA Mutations (PI3Kinase)
Studies have also found that mutations in the PI3KCA gene range from 0–5 % of patients with EGFR TKI-acquired resistance.7,45,69 The PI3K cell growth and survival pathway is downstream of EGFR and therefore targeting the PI3K pathway could potentially have a synergistic effect when used with an EGFR inhibitor.44 Two clinical trials are currently investigating the pan-class I PI3K inhibitor buparlisib (BKM120) in combination with erlotinib (phase II, NCT01487265) or gefitinib (phase Ib, NCT01570296) in EFGR TKI-resistant aNSCLC.
Biopsy and Molecular Testing at Disease Progression
According to the NCCN, reevaluation with biopsy upon disease progression is reasonable for the identification of patients with actionable mutations or those who may have SCLC histology.2 However, a recent NCCN survey indicates that only ~27 % of clinicians generally conduct a biopsy at disease progression.75 This low percentage may be explained by perceived barriers associated with biopsy at disease progression (e.g., patient unwillingness, AEs associated with the biopsy procedures, insufficient tissue acquisition).76 Additionally, physicians may be less willing to test without having approved targeted therapies available. According to one prospective multicenter study (GFPC study 12-01) that assessed the feasibility and clinical utility of biopsy upon disease progression in aNSCLC, >90 % of the time patients are willing to undergo biopsy.77 Of the 82 % of patients with a biopsy upon progression, 94 % and 74.4 % of tumors can be histologically and molecularly analyzed, respectively.77 Two separate studies reported low (<5 %) biopsy-related AEs at progression.69,77 Based on these findings it is critical that the mechanisms of resistance are identified at the point of disease progression in order to guide subsequent therapeutic choices.
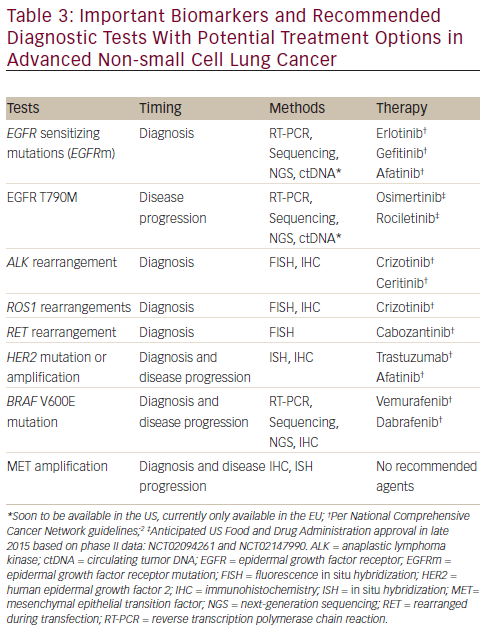
Tissue biopsy at progression requires collaboration among oncologists, interventional radiologists, and/or pulmonologists and pathologists.78 The types of testing will vary by institution and practice, but may include commercially available kits (eg, cobas® EGFR Mutation Test, Roche Diagnostics, Indianapolis, IN; and FoundationOne® testing, Foundation Medicine, Inc., Cambridge, MA) or laboratory-developed tests. Other molecular tests include immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS).79 Table 3 lists the types of tests that are routinely ordered for aNSCLC molecular testing and includes tests for both biopsy at initial diagnosis and biopsy at disease progression.
Recent advances in noninvasive approaches such as plasma testing, or “liquid” biopsy, provide an exciting alternative to molecular testing.80 Due to the moderate sensitivity of current circulating tumor DNA (ctDNA) assays (range, 46–81 %), it is important to note that if a test result is negative a tissue biopsy may still be required to rule out a false-negative.12,81–83 However, improvements to ctDNA testing are underway that enhance detection, including more sensitive reverse transcription polymerase chain reaction (RT-PCR) and the development of newer methods, such as digital PCR.82,84
ctDNA testing is available in Europe as an assessment for EGFRm status and it may be used where no tumor sample is available, or as a means to monitor disease progression. This test is highly predictive of EGFRm tumors.12,80,81,85 ctDNA testing can also identify the EGFR T790M resistance mutation.83 Albeit preliminary, new developments such as EGFR mutational analysis from urine samples are on the horizon.86,87
Conclusions
Choice of first-line therapy for EGFRm-positive aNSCLC should be based on multiple factors, including efficacy (e.g., EGFRm ex19del, L858R, and rare mutations), tolerability profiles, and costs. Unfortunately, most patients’ disease will progress on EGFR TKI therapy, highlighting the need to test for mechanisms of resistance and to develop targeted agents. Multiple mechanisms of resistance have been identified, with the most common being the EGFR T790M mutation. Targeted therapies for EGFR T790M, such as osimertinib and rociletinib, will provide crucial options for these patients. Based on the clinical trial data to date, efficacy seems similar for the two agents. However their tolerability profiles differ with different rates of rash and diarrhea, and high rates of hyperglycemia observed with rociletinib. Therefore, choosing which option to use will depend on the ease of administration, dosing schedule, compliance factors, tolerability profile, and cost.
Before osimertinib and rociletinib, chemotherapy was also an important option when choosing a therapy at the time of progression after firstline EGFR TKI treatment, due to its benefits to response rate and PFS. However, patients who have T790M-positive aNSCLC may potentially be treated with third-generation EGFR TKIs, such as osimertinib or rociletinib. Therefore, biopsy at progression on EGFR TKI is strongly recommended to evaluate the mechanism of resistance and guide the selection of targeted therapies. In addition to traditional biopsy methods, “liquid” biopsy or plasma ctDNA could potentially help identify these resistance mechanisms in some patients. This is an exciting time for personalized treatments for aNSCLC, as more research is dedicated to understanding each of these mechanisms of progression and the novel ways to detect and treat them.