Outcomes for patients with metastatic colorectal cancer have steadily improve due to discovery of new drugs as well as increasing number patients undergoing local therapies.1Expand Reference Angiogenesis is of a paramount importance in this disease and since vascular endothelial growth factor (VEGF) discovery several drugs targeting this pathway have been developed.2,323
Fruquintinib (HMPL-013) is a tyrosine kinase inhibitor (TKI) that primarily targets vascular endothelial growth factor receptor (VEGFR) with a minor activity in other ancillary targets, such as fibroblast growth factor receptor 1 (FGFR1), platelet-derived growth factor receptor (PDGFR), as well as CD117 (cluster of differentiation 117, also known as KIT).4Expand Reference
The drug was developed by Hutchison MediPharma (Shanghai, China) and then co-developed and commercialized by Eli Lilly (Indianapolis, IN, USA) in 2013.5 Expand ReferenceIn 2018, fruquintinib was approved by China’s National Medical Products Administration (formerly the China Food and Drug Administration) for the treatment of patients with metastatic colorectal cancer (mCRC) based on the phase III FRESCO (A randomized, double-blind and placebo-controlled phase III trial comparing fruquintinib efficacy and safety vs best support care (BSC) in advanced colorectal cancer patients who have failed at least second lines of chemotherapies; ClinicalTrials.gov identifier: NCT02314819) trial.6Expand Reference More recently, the FRESCO-2 trial (A global multicenter randomized placebo-controlled phase 3 trial to compare the efficacy and safety of fruquintinib plus best supportive care to placebo plus best supportive care in patients with refractory metastatic colorectal cancer; ClinicalTrials.gov identifier: NCT04322539) demonstrated the benefit of fruquintinib versus placebo in patients with mCRC previously treated with all standard therapies, including trifluridine/tipiracil (FTD/TPI, TAS-102) and/or regorafenib.7Expand Reference The US Food and Drug Administration (FDA) has granted a priority review of the New Drug Application for fruquintinib in mCRC.8Expand Reference
In this article, we are going to summarize this trajectory, as well as point out possible approaches to be explored in the future.
Preclinical studies
In vitro, fruquintinib inhibited VEGFR family kinases and suppressed vascular endothelial growth factor (VEGF)/VEGFR cell signalling in human umbilical vein endothelial cells and human lymphatic endothelial cells with a half-maximal inhibitory concentration (IC50) at a low nanomolar level. This small molecule is a potent VEGFR inhibitor with a high kinome selectivity, targeting VEGFR-1, VEGFR-2 and VEGFR-3 (Figure 1), while it has a minor inhibitory effect on other targets, such as c-kit, PDGFR, rearranged during transfection (RET) and FGFR-1.4,545
Figure 1: The vascular endothelial growth factor receptor family and fruquintinib activity
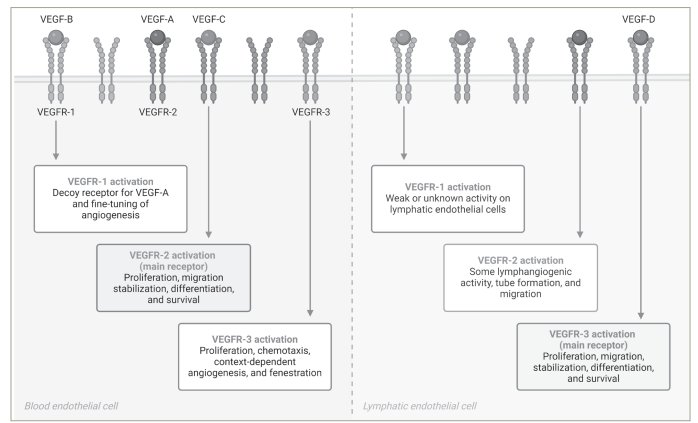
Created with BioRender.com.
VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor.
In the meantime, angiogenesis exerts an immunosuppressive function through diverse mechanisms, including the direct suppression of antigen-presenting cells and immune effector cells. This also involves the upregulation of regulatory T cells (Treg), myeloid-derived suppressor cells (MDSCs) and tumour-associated macrophages. Anti-angiogenic agents, such as fruquintinib, have emerged as an option to modulate the tumour microenvironment (TME).9Expand Reference
In vivo, anti-tumour activity was evaluated in several different xenograft models, and plasma concentration measurements have supported the correlation between drug exposure and anti-tumour activity.10Expand Reference Sun et al. proved preclinical activity using different mice models of different tumors and results from the gastric cancer model (BGC-823), for example, demonstrated that the drug concentration needs to be at least maintained above the drug concentration required to inhibit the receptor phosphorylation by 85% (EC85 ) for around 8 h in order to achieve >80% tumour growth inhibition.10Expand Reference
The molecule also demonstrated favourable pharmacokinetic attributes, including rapid absorption, high oral bioavailability, gradual excretion, reasonable distribution within tissues and a low propensity for drug–drug interactions.4Expand Reference Furthermore, fruquintinib has demonstrated enhanced anti-tumour activity when combined with cytotoxic chemotherapy with a reasonable tolerance in different patient-derived xenograft models.10Expand Reference Finally, using two human colorectal cancer (CRC) xenografts in nude mouse models (SW48 and HCT 116), fruquintinib has demonstrated a synergism when combined with FTD/TPI.11Expand Reference
Numerous TKIs have been formulated for the treatment of solid tumours. However, their efficacy at the maximum tolerated dose (MTD) is constrained due to the broader spectrum of kinase inhibition, which might lead to heightened toxicity and/or suboptimal inhibition of a specific target. When compared with regorafenib, for example, fruquintinib showed a higher selectivity to VEGFR (Table 1).5,12512 In fact, few kinases other than VEGFRs were inhibited in a panel of 253 kinase tests, and this could potentially minimize off-target toxicity and allow a higher exposure at MTD.5,12512
Table 1: Half-maximal inhibitory concentration (mmol/L)5,12512
|
|
VEGFR-1 |
VEGFR-2 |
VEGFR-3 |
PDGFR-α |
PDGFR-β |
FGFR |
c-KIT |
|
Regorafenib5Expand Reference |
13 |
4.2 |
46 |
NS |
22 |
NS |
7 |
|
Fruquintinib12Expand Reference |
33 |
35 |
0.5 |
NS |
NS |
NS |
NS |
c-KIT = KIT Proto-Oncogene, Receptor Tyrosine Kinase; FGFR = fibroblast growth factor receptor; NS = not significant; PDGFR = platelet-derived growth factor receptor; VEGFR = vascular endothelial growth factor receptor.
Clinical studies in metastatic colorectal cancer
As previously evidenced in pivotal trials of regorafenib and FTD/TPI, initial studies exploring fruquintinib in the context of refractory mCRC demonstrated tolerable toxicity and moderate efficacy, leading to the development of a pivotal phase III trial (FRESCO) (Table 2).5–7,13,145671314
Table 2: Clinical studies with fruquintinib in metastatic colorectal cancer5–7,1356713
|
Clinical trial identifier |
Phase |
Line |
N |
mOS with fruquintinib (months) |
mOS with control (months) |
mPFS with fruquintinib (months) |
mPFS with control (months) |
ORR with fruquintinib (%) |
ORR with control (%) |
|
NCT019750775Expand Reference |
Ib |
Third |
42 |
8.88 |
NA |
5.8 |
NA |
9.5 |
NA |
|
NCT0219668813Expand Reference |
II |
Third |
71 |
7.72 |
5.52 |
4.73 |
0.99 |
2.1 |
0 |
|
NCT02314819 (FRESCO)6Expand Reference |
III |
Third |
416 |
9.3 |
6.6 |
3.7 |
1.8 |
4.7 |
0 |
|
NCT04322539 (FRESCO-2)7Expand Reference |
III |
Later |
691 |
7.4 |
4.8 |
3.7 |
1.8 |
1.5 |
0 |
mOS = median overall survival; mPFS = median progression-free survival; ORR = objective response rate; NA = not applicable.
The pharmacokinetic data presented in the phase I clinical trial confirmed that plasma exposure at MTD was able to achieve an EC85 for 24 h, providing the rationale for a once-daily regimen.12Expand Reference
Phase III FRESCO trial
FRESCO was a randomized, double-blind, placebo-controlled, multicentre, phase III clinical trial, which randomized 416 patients in 28 hospitals in China.6Expand Reference In this study, participants who had received at least two lines of chemotherapy were randomized in a 2:1 ratio to fruquintinib (5 mg orally once daily, 3 weeks on, followed by 1 week off) or placebo.
The FRESCO trial met its primary endpoint: the median overall survival (mOS) was 9.30 months (95% confidence interval [CI] 8.18–10.45) with fruquintinib versus 6.57 months (95% CI 5.88–8.11) with placebo (hazard ratio [HR] for death 0.65 [95% CI 0.51–0.83]; p<0.001). The secondary endpoint, the median progression-free survival (mPFS), was also significantly increased (3.71 months [95% CI 3.65–4.63] with fruquintinib versus 1.84 months [95% CI 1.81–1.84] in the placebo arm; HR 0.26 [95% CI 0.21–0.34]; p<0.001). As previously seen with other agents in later lines, the objective response rate was modest with fruquintinib: 4.7% versus 0% (p=0.01). The disease control rate (DCR) was 62.2% with fruquintinib versus 12.3% with placebo (p<0.001).
On the other hand, toxicity was not trivial. Grade 3–4 hypertension occurred in 21.2% of participants, hand–foot syndrome (HFS) in 10.8% and proteinuria in 3.2%. In addition, these toxicities led to a treatment interruption or dose reduction in 47.1% of fruquintinib-treated patients versus 13.1% with placebo. Among them, the most common cause of discontinuation or dose reduction was HFS (13.3%), proteinuria (9.7%) and thrombocytopaenia (5.4%).
Phase III FRESCO-2 trial
The FRESCO-2 trial was another pivotal study which enrolled patients who had an mCRC and received prior chemotherapy comprising fluoropyrimidine, oxaliplatin or irinotecan; a VEGF inhibitor and an epithelial growth factor receptor inhibitor if they had a rat sarcoma virus gene wild-type disease.7Expand Reference In addition, to be eligible, patients were required to have experienced disease progression on or been intolerant to FTD/TPI (TAS-102) and/or regorafenib. The differences in eligibility criteria and study population between both phase III trials are highlighted in Table 3.6,767
Table 3: Comparison between FRESCO and FRESCO-2 trials6,767
|
|
FRESCO6Expand Reference (%) |
FRESCO-27Expand Reference (%) |
|
Prior therapies |
|
|
|
VEGF inhibitor |
30 |
97 |
|
EGFR inhibitor |
25 |
39 |
|
TAS-102 |
0 |
52 |
|
Regorafenib |
0 |
9 |
|
TAS-102 and regorafenib |
0 |
39 |
|
Study population |
|
|
|
USA |
0 |
18 |
|
Spain |
0 |
26 |
|
Italy |
0 |
16 |
|
Other European countries |
0 |
30 |
|
Japan |
0 |
8 |
|
Australia |
0 |
3 |
|
China |
100 |
0 |
EGFR = epithelial growth factor receptor; RAS = rat sarcoma virus gene; VEGF = vascular endothelial growth factor.
The mOS was 7.4 months (95% CI 6.7–8.2) with the fruquintinib group versus 4.8 months (95% CI 4.0–5.8) in the placebo arm (HR 0.66 [95% CI 0.55–0.80]; p<0.0001). mPFS and DCR were also increased in the interventional arm: 3.7 versus 1.8 months (HR 0.32 [95% CI 0.27–0.39]; p<0.0001) and 55.5% versus 16.1 % (p<0.0001). Unfortunately, no biomarker is available to predict the response to the agent, which is obviously a limitation of both studies, bringing additional difficulties to the decision-making process in a scenario with at least two other options available.
Again, treatment-emergent adverse effects (TEAEs) of any grade were experienced by 98.9% of patients with fruquintinib versus 92.6% with placebo, with grade 3 or worse toxicities reported in 62.7 and 50.4% of patients, respectively. The most frequent grade 3 or worse TEAEs were hypertension (13.6 versus 0.9%), asthenia (7.7 versus 3.9%), reduced appetite (2.4 versus 1.3%), diarrhoea (3.5 versus 0%), hypothyroidism (0.4 versus 0%), fatigue (3.9 versus 0.9%) and HFS (6.4 versus 0%) in the fruquintinib and placebo arms, respectively. Moreover, dose interruptions occurred in 47% of patients and dose reductions occurred in 24% of patients who were treated with fruquintinib, which compares favourably with regorafenib and is promising, particularly considering the later-line setting of these patients. A similar proportion of patients in the fruquintinib and placebo groups discontinued the treatment due to adverse events, 20 versus 21%, respectively.7Expand Reference
On-going studies in colorectal cancer
Inhibiting neoangiogenesis elicits vascular normalization and prompts immune reprogramming, thereby counteracting the immunosuppressive milieu of the TME and rendering fruquintinib a compelling candidate for synergistic deployment alongside immune checkpoint inhibitors (ICIs). Acknowledging that VEGF plays a critical role in TME modulation, through T cells, Treg, dendritic cells and MDSCs, fruquintinib could be a better partner to ICIs compared with other TKIs that have failed in CRC, such as lenvatinib and regorafenib, due to its selectivity.
Analogous to the scenario witnessed with regorafenib, multiple clinical trials are currently investigating the potential of fruquintinib in conjunction with cytotoxic chemotherapy (see Table 4).15–29 151617181920...29Regorafenib, a multitargeted kinase inhibitor with a focus on angiogenic and stromal receptor tyrosine kinases, has exhibited a modest benefit when administered alongside 5-fluorouracil and oxaliplatin (FOLFOX) regimen in cases of gastro-oesophageal cancer.30Expand Reference Another anti-angiogenic agent, bevacizumab, when combined with FTD/TPI, led to the normalization of blood vessels, leading to an increased FTD content in tumours as well as to clinically meaningful benefit responsible for the FDA approval.31Expand Reference Given the previous benefit demonstrated in the SUNLIGHT study (An open-label, randomized, phase III study comparing trifluridine/tipiracil in combination with bevacizumab to trifluridine/tipiracil monotherapy in patients with refractory metastatic colorectal cancer; ClinicalTrials.gov identifier: NCT04737187) and the absence of overlapping toxicity, a combination of FTD/TPI and fruquintinib has become a promising alternative.31Expand Reference Obviously, considering the undeniable importance of angiogenesis in the context of mCRC, the promise of these combinations is discernible, although concerns surrounding the potential toxicities remain unsolved.
Table 4: On-going studies with fruquintinib in colorectal cancer15–29151617181920...29
|
|
Clinical trial identifier |
Phase |
Treatment |
Primary endpoint |
|
First line |
|
|
|
|
|
|
NCT0197507716Expand Reference |
II |
FOLFOX/FOLFIRI and fruquintinib |
PFS |
|
Maintenance |
|
|
|
|
|
|
NCT0429601917Expand Reference, NCT0501686918Expand Reference, NCT0545171919Expand Reference, NCT0473396320Expand Reference and NCT0565929021Expand Reference |
II or I/II |
Fruquintinib or fruquintinib plus capecitabine |
PFS |
|
Second line |
|
|
|
|
|
|
NCT0563459022Expand Reference |
II |
FOLFOX/FOLFIRI and fruquintinib |
PFS |
|
|
NCT0555590123Expand Reference |
II |
FOLFIRI plus fruquintinib versus FOLFIRI plus bevacizumab |
PFS |
|
|
NCT0552273824Expand Reference |
Ib/II |
FOLFIRI and fruquintinib |
ORR |
|
|
NCT0544771525Expand Reference |
II |
Fruquintinib sequential bevacizumab plus FOLFIRI versus bevacizumab plus FOLFIRI sequential fruquintinib |
PFS |
|
Third line |
|
|
|
|
|
|
NCT0500483126Expand Reference |
II |
Fruquintinib and trifluridine/tipiracil |
PFS |
|
Chemorefractory |
|
|
|
|
|
|
NCT0469547027Expand Reference |
II |
Fruquintinib and sintilimab |
PFS |
|
|
NCT0458298128Expand Reference |
II |
Fruquintinib plus raltitrexed versus fruquintinib |
PFS |
|
|
NCT0486686229Expand Reference |
II |
Fruquintinib and camrelizumab |
ORR |
Evaluation of fruquintinib in the continuum of care of patients with colorectal cancer © 2023 by Daniele Lavacchi is licensed under CC BY 4.0
FOLFIRI = 5-fluorouracil and irinotecan; FOLFOX = 5-fluorouracil and oxaliplatin; ORR = objective response rate; PFS = progression-free survival.
Incorporation of fruquintinib
The question remains as to the choice of third-line therapy for mCRC. There are no clinical trials that directly compare the efficacy and safety among fruquintinib, regorafenib and FTD/TPI. At the time of the FRESCO trial, the standard practices for mCRC in China were not the same as those outside of China.32Expand Reference Only 30% of patients had received VEGF-targeted therapy, and none had received FTD/TPI or regorafenib. As neither FTD/TPI nor regorafenib was available, a placebo was used as the control.6Expand Reference In comparison, over 96% of patients in the FRESCO-2 study had received a VEGF inhibitor, and all patients were required to have had disease progression on or been intolerant to FTD/TPI or regorafenib.7Expand Reference Therefore, it is still difficult for clinicians to decide on the best treatment option for patients who have failed at least two lines of systemic therapy. A meta-analysis incorporating the CORRECT (A randomized, double-blind, placebo-controlled phase III study of regorafenib plus BSC versus placebo plus BSC in patients with metastatic colorectal cancer [CRC] who have progressed after standard therapy; ClinicalTrials.gov identifier: NCT01103323), CONCUR (A randomized, double-blind, placebo-controlled phase III study of regorafenib plus best supportive care [BSC] versus placebo plus BSC in Asian subjects with metastatic colorectal cancer [CRC] who have progressed after standard therapy; ClinicalTrials.gov identifier: NCT01584830), RECOURSE (Randomized, double-blind, phase 3 study of TAS-102 plus best supportive care (BSC) versus placebo plus BSC in patients with metastatic colorectal cancer refractory to standard chemotherapies; ClinicalTrials.gov identifier: NCT01607957), TERRA (Randomized, double-blind, phase III study of TAS-102 versus placebo in Asian patients with metastatic colorectal cancer refractory or intolerable to standard chemotherapies; ClinicalTrials.gov identifier: NCT01955837) and FRESCO trials, with a total of 2,586 patients reported similar overall survival (OS) and progression-free survival (PFS) between fruquintinib and regorafenib and between regorafenib and FTD/TPI; however, fruquintinib, compared with FTD/TPI, showed superior PFS but similar OS.7,14,33–3671433343536
Few retrospective studies conducted in China reported similar efficacy and toxicity profiles between fruquintinib and regorafenib; however, regorafenib followed by fruquintinib showed longer OS than the reverse treatment sequence.37,383738 On the other hand, a meta-analysis including three randomized trials and 1,380 patients showed a significant difference in all-grade toxicity compared with regorafenib (odds ratio [OR] 0.73; 95% CI 0.65–0.82) with no difference in grade 3–5 events (OR 0.92; 95% CI 0.64–1.32).39Expand Reference This difference in lower toxicity with fruquintinib might be due to the higher selectivity in inhibiting angiogenesis. Further clinical investigation will be needed to determine the optimal treatment sequence.
Conclusions
Substantial evidence from phase III trials has established fruquintinib as a new standard therapy in refractory mCRC, underscoring neoangiogenesis as one of the most critical processes for tumour progression.
While every effort should be made to identify clinical and molecular biomarkers predictive of response, as previously seen with regorafenib in a similar scenario, toxicity was conspicuous and studies with dose reduction should be encouraged.
Finally, considering the modulation in the TME, fruquintinib emerges as a compelling option to combine with different ICIs as well as with cytotoxic chemotherapy, and several trials are on-going exploring these combinations.