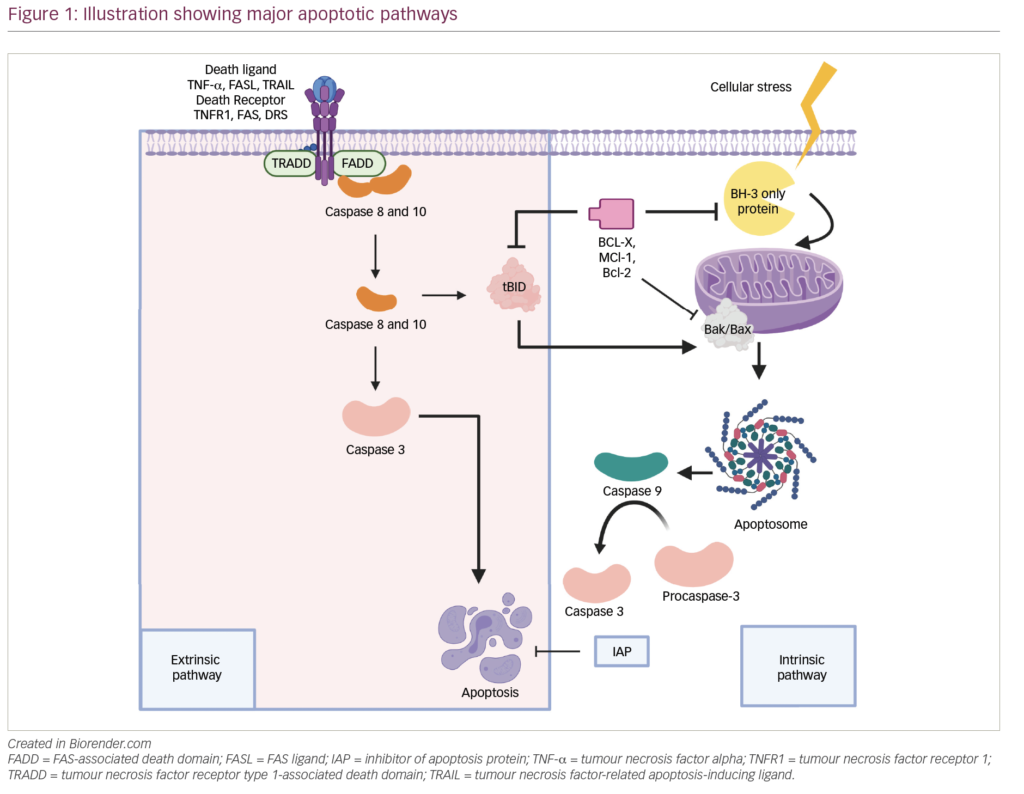
The disease results from malignant plasma cells, which infiltrate the bone marrow.Although much is known about the nature of MM, the causes remain unknown. Genetic abnormalities are common and varied.2 The disease is predominantly localized to the bone marrow.The malignant plasma cells adhere to bone marrow stromal cells (BMSCs), resulting in the up- regulation of cytokines such as interleukin (IL)-1_,IL-6, insulin-like growth factor (IGF)-1, and vascular endothelial growth factor (VEGF).3 These cytokines are important for the regulation of plasma cell proliferation and migration, as well as for the development of drug resistance in myeloma. CD40 is present on MM plasma cells and BMSCs. CD40 ligand, present on T-cells, increases endogenous IL-6 secretion by MM cells and BMSCs through activation of nuclear factor-kappa B (NF-ggB),4 and increases expression of bcl-2, which increases drug resistance. Chemotherapy itself induces drug resistance by activation of NF-ggB5 and up- regulation of bcl-2 in MM cells.6
The clinical manifestations of MM result predominantly from marrow failure, immunosuppression, lytic lesions of the axial skeleton and long bones, and renal failure. Anemia occurs in the majority of myeloma patients, secondary to plasma cell infiltration and anemia of chronic disease.The overall median survival is three years. When patients are diagnosed with MM,chemotherapy is usually initiated to relieve symptoms and to prolong survival, but cures are rare. Therefore, with current standard treatment strategies the goals are prolongation of survival and improvement of symptoms. Recent years have seen a major change in the treatment of MM. By understanding the biology of malignant disease and defining important potential treatment targets, effective new drugs can be developed to improve patient outcome in MM.This article provides only a brief summary of some of the major recent developments in biologically based therapy for MM. As an increasing number of targets are being identified and mechanisms of resistance are elucidated, combinations of drugs will be developed to optimize new therapeutic combinations.
Conventional Chemotherapy
Untreated, the average survival of patients with MM is approximately six months.Although low doses of oral melphalan with prednisone has extended the median survival of MM from six months to three years, the response rates to melphalan and prednisone have been only 50% to 60%. Complete remissions are rare, and myeloma remains incurable with conventional chemotherapy. Overall survival has not been significantly improved with vincristine, adriamycin, and dexamethasone (VAD), or other forms of infusional chemotherapy, over melphalan and prednisone.7 The bone marrow toxicity of conven- tional chemotherapy is dose-limiting. Hematopoietic cell transplantation, or marrow transplantation, with either a patientýs own marrow (autologous) or a family member (allogeneic) has been explored extensively as a means to increase doses of conventional chemotherapy.
Allogeneic transplantation has been associated with a very high treatment-related mortality of up to 40%, and therefore has been performed infrequently. In contrast to allogeneic transplantation, autologous transplantation has been much safer, with a treatment- related mortality of <3%.Autologous transplantation is superior to conventional chemotherapy for the treatment of MM, and has been associated with a median survival to 55ý72 months.8 However, only 50% of patients aged 60 or under, and fewer than 20% of patients between 60 and 70, receive transplantation due to early death, co-morbidity, poor response to therapy, and failed peripheral blood stem cell harvests.9 For these patients, new therapies are urgently needed. For those who do receive marrow transplantation, improved patient outcome has been associated with increased remission rates and prolonged survival, but cures remain elusive.New strategies to control minimal residual disease after marrow transplantation are needed. Over the last decade, major advances in understanding the biology of MM have led to significant advances in treatment that are aimed at new cellular molecular targets. Novel therapies may improve response to marrow transplantation and may improve the overall survival of all MM patients with or without transplantation and, in contrast to transplan- tation, should be feasible for most patients.
Novel Biologically Based Therapies
Recently, there have been major new developments in novel biologically based therapies for MM. In particular, proteasome inhibitors, thalidomide and immuno- modulatory analogs of thalidomide, 2-methoxyestradiol, arsenic trioxide, monoclonal antibodies, and histone deacetylase inhibitors have entered into clinical trials for MM. Although these novel therapies were initially evaluated primarily in patients who relapse after transplantation, due to their efficacy and tolerability, some of these agents are now being evaluated in earlier stage patients, as initial therapy or treatment of first relapse, and in combination with other therapies. The examples presented in this review represent the beginning of a new age of translational research, when understanding the molecular biology of malignant disease can be translated into a favorable therapeutic outcome for patients.
Proteasome Inhibition
The 26S proteasome is a large ubiquitous multi- protein complex comprised of a 20S core catalytic particle flanked by two 19S regulatory particles.This protein complex is essential to regulating diverse cellular functions including cell proliferation and division, apoptosis, and resistance to drug-induced cell death. Furthermore, the proteasome is involved in degrading abnormally folded or oxidized proteins.The first proteasome inhibitor to enter clinical trials was a boronic acid dipeptide originally called PS-341, but since renamed Velcade, or bortezomib.10 Velcade ushered in a new era of drug development, being the first drug in a new class of compounds to reach clinical trials, and subsequently the first new drug in over three decades to be US Food and Drug Administration (FDA)-approved in the US for the treatment of MM. In vitro and in vivo pre-clinical experiments demonstrated significant activity against MM cells.11,12 Actively dividing cells such as malignant cells appear more sensitive to proteasome inhibition than quiescent or differentiated cells such as endothelial cells. However, other events also contribute to apoptosis induced by proteasome inhibition, such as inhibition of NF-gB and modulation of the bone marrow micro-environment in MM patients.3 In a multicenter, open-label, non- randomized, phase 2 trial, 202 patients with relapsed and refractory MM received 1.3mg/m2 bortezomib twice-weekly for two weeks, followed by one week without treatment, for up to eight cycles (24 weeks).13 In patients with a sub-optimal response, oral dex 20mg daily was added to the regimen.The response rate was 35%, and 68% of patients had either decreased or stable paraprotein levels. The median duration of response was 12 months, and median overall survival was 16 months. This was a significant improvement over an historical median survival of nine months in patients with relapsed and refractory MM. Grade 3 adverse events included thrombocytopenia (28%), fatigue (12%), peripheral neuropathy (12%), and neutropenia (in 11%). Grade 4 events occurred in 14%. In May 2003, Velcade was the first-in-class proteasome inhibitor to be approved by the FDA for use in patients with relapsed refractory MM. As a single agent,Velcade represents a significant advance in the treatment of MM. A phase 3 clinical trial, comparing Velcade with dex for treatment of relapsed MM, is now on-going. Clinical trials are also underway to explore the activity of Velcade when combined with conventional therapy such as doxorubicin or melphalan.
Thalidomide and Analogs
The anti-angiogenic properties of thalidomide, together with the increased angiogenesis observed in the marrow of patients with MM, led to the use of thalidomide in MM in the late 1990s. Although thalidomide was initially used for its anti-angiogenic properties, subsequent investigations have revealed that thalidomide also has direct cytotoxic activity against MM cells in vitro. Single agent thalidomide in MM achieved a response rate of over 30%, predominantly in patients with refractory disease following autologous transplantation.14 It has been shown in vitro that the addition of dex to thalidomide enhances anti-myeloma activity.15 In clinical trials, the response rate was 36% for patients treated with thalidomide alone and 72% for patients treated with the combination of thalidomideýdex, including 16% complete remission in the thalidomideýdex-treated group.16 Significant anti-myeloma activity has been observed even after prior resistance to thalidomide and dex given separately, as well as after intensive therapy supported by autologous hematopoietic cell transplant (AHCT). However, at least one third of patients treated with thalidomide have experienced mild or moderate constipation, fatigue, or somnolence. More potent thalidomide analogs have been developed that have a much more favorable side effect profile, and these may have an even greater impact on response rates to induction chemotherapy while minimizing toxicity of therapy.
Immunomodulatory Thalidomide Analogs
Immunomodulatory drugs (IMiDs) such as CC-5013 (Revlimid) are potent thalidomide analogs that markedly stimulate T-cell proliferation, as well as IL-2 and interferon (IFN)-a production.17 Revlimid is 50 to 2,000 times more potent than thalidomide in stimulating T-cell proliferation triggered via the T-cell receptor and 50 to 100 times more potent than thalidomide in augmenting IL-2 and IFN-aa production. Revlimid inhibits tumor growth, decreases angiogenesis, and prolongs host survival in a human plasmacytoma mouse model.18 In myeloma patients, Revlimid has been shown to stimulate host anti-MM natural killer (NK) cell immunity,19 and augment antibody-dependent cellular cytotoxicity in vitro.20 Importantly, a phase I study of Revlimid reported no significant somnolence, constipation, or neuropathy in any cohort.21 At least a 25% reduction in paraprotein was achieved in 17 (71%) of 24 patients, and 4% achieved complete remission. This anti-MM activity was particularly impressive because 15 patients (60%) had undergone prior AHCT, and 16 patients (64%) had progressive disease despite treatment with thalidomide. The evaluation of Revlimid has rapidly forged ahead to a phase II multi-center trial.22
2-Methoxyestradiol
2-Methoxyestradiol (2ME2) is an estrogen derivative, but induces cell death independently of the estrogen receptor. 2ME2 mediates anti-MM activity directly on MM cells and in the bone marrow (BM) micro- environment.23 Although it is a corticosteroid-derived compound, death signaling induced by 2ME2 differs from the corticosteroid dex. Furthermore, 2ME2 induces apoptosis in MM cells resistant to dex. 2ME2- induced apoptosis is mediated by the release of mitochondrial cytochrome-c and Smac, followed by the activation of caspases-8, -9, and -3. In contrast to its effects on MM cells, 2ME2 does not reduce the survival of normal peripheral blood lymphocytes. Moreover, 2ME2 enhances dex-induced apoptosis, and its effect is not blocked by IL-6. In a murine model, 2ME2 inhibited MM cell growth, prolonged survival, and decreased angiogenesis.A multicenter phase II trial for 2ME2 in patients with chronic-phase MM is currently underway in the US.
Arsenic Trioxide
In the early 1990s, impressive results were observed with arsenic trioxide in patients with acute promyelocytic leukemia (APL), leading to the approval of its use in the US for relapsed or refractory APL.
Subsequent pre-clinical studies showed significant activity of arsenic trioxide in MM.24 Arsenic induces superoxide production while reducing glutathione levels, resulting in mitochondrial-mediated apoptosis.25 The activity of arsenic may be enhanced by ascorbic acid-mediated cellular glutathione depletion. In a phase I/II trial of six patients with stage IIIA relapsed/ refractory myeloma, 0.25mg/kg/day of arsenic together with 1,000mg/day of ascorbic acid was given for 25 days over a 35-day period without dose-limiting toxicity.26 Only one episode of grade 3 hematological toxicity (leukopenia) and no grade 3 non- hematological toxicities were observed. Two patients (both with thalidomide-refractory disease) had partial responses; four patients had stable disease.Therefore, the combination of arsenic together with ascorbic acid has acceptable toxicity and is active in refractory/relapsed myeloma. Arsenic with ascorbic acid is now being evaluated in combination with either melphalan or dex in separate phase II studies in the US.
Histone Deacetylase Inhibitors
Histone deacetylase (HDAC) inhibitors are a promising new treatment strategy in hematologic malignancies.27,28 Gene expression, cellular differentiation, and survival are regulated by the opposing activities of histone acetyltransferases (HATs) and HDACs. HDAC inhibition results in accumulation of acetylated nuclear histones and induces differentiation and/or apoptosis in transformed cells. HDAC inhibitors mediate anti-MM activity and overcome drug resistance in the BM milieu. They sensitize MM cells to death receptor- mediated apoptosis, and inhibit the secretion of IL-6 induced in BMSCs by binding of MM cells, thus overcoming cell adhesion-mediated drug resistance.
HDAC inhibitors are synergistic with dex, bortezomib, and IGF-1 inhibitors against MM, and may one day be incorporated into combinations of novel therapies and conventional therapies in the treatment of MM.
Anti-CD40 Monoclonal Antibody
Humanized anti-CD20 monoclonal antibody Rituximab has been useful in the treatment of non- Hodgkinýs lymphoma. MM cells do not express significant levels of CD20, but CD40, which is expressed on B-cell malignancies, is also expressed on human MM cells. CD40 stimulation up-regulates VEGF secretion and MM cell migration.29,30 SGN-40 is a humanized anti-CD40 monoclonal antibody, which shows pre-clinical activity against human MM cell lines (HMCLs) and patient MM cells expressing CD138 and CD40.31 SGN-40 induces apoptosis in dex-sensitive MM.1S and dex-resistant MM.1R cells, and in patient MM cells. SGN-40-mediated cytotoxicity is associated with up-regulation of cytotoxic ligands of the tumor necrosis factor (TNF) family (Fas/FasL, TNF-related apoptosis-inducing ligand, and TNF-__), and pre-treatment of MM cells with SGN-40 blocks CD40 ligand-mediated phosphatidylinositol 3ý-kinase/AKT and NF-gB activation, events associated with cellular survival and drug resistance. Importantly, pre-treatment of MM.1S and MM.1R cells with SGN-40 inhibits proliferation triggered by IL-6. In addition, SGN-40 pre-treatment of MM.1S cells blocks the ability of IL- 6 to protect against dex-induced inhibition of DNA synthesis. A phase I, multi-dose study of SGN-40 in patients with refractory or relapsed MM has begun accrual at the Dana-Farber Cancer Institute.
VEGF
Human myeloma cell lines and MM patient cells express high-affinity VEGF receptor-1 or Fms-like tyrosine kinase-1 (Flt-1). VEGF inhibitors PTK78732 and GW65465233 are novel therapies currently under pre-clinical and clinical evaluation for the treatment of MM. These small molecule tyrosine-kinase inhibitors block VEGF-induced signaling and migration in MM cells that are both sensitive and resistant to conventional therapy. Importantly, GW654652 also inhibits IL-6 and VEGF secretion and proliferation of MM cells induced by tumor cells binding to BMSCs. The activity of a pan-VEGF receptor inhibitor against MM cells in the BM milieu, coupled with its lack of major toxicity in pre-clinical mouse models, provides the framework for clinical trials of this drug class to improve patient outcome in MM.
Insulin-like Growth Factor-1
Insulin-like growth factors and their receptor (IGF-1R) have been implicated in cancer pathophysiology. IGF-1R is expressed in solid tumors and various hematologic malignancies, including MM. Specific IGF-1R inhibition with neutralizing antibody, antagonistic peptide, or the selective kinase inhibitor NVP-ADW742 have in vitro activity against MM, including those resistant to conventional therapies. NVP-ADW742, alone or in combination with cytotoxic chemotherapy, has shown significant anti-tumor activity in a xenograft MM murine model, providing the rationale for the therapeutic use of selective IGF-1R inhibitors in MM.34
Conclusion
This summary has provided an overview of some of the major recent developments in the approach to biologically based therapy for MM. However, an increasing number of targets are being identified and more potential drugs are being developed every day. As mechanisms of resistance are elucidated, combinations of drugs will be developed to optimize new drug combinations and improve patient outcome in MM.