Multiple myeloma (MM) is an incurable disease characterised by accumulation of clonal plasma cells in the bone marrow and accounts for approximately 10 % of all haematological malignancies.1 Advances in the understanding of MM, better identification of high-risk patients, and the recent development of several novel agents have improved response and nearly doubled the length of time to disease
progression, leading to overall survival (OS) on the order of 7 to 10 years.2–5 However, since almost all patients eventually relapse and become refractory to current standards of care, there is a constant need for effective new therapies and treatment regimens. Indeed, prognosis for patients who have become refractory to proteasome inhibitors (e.g., bortezomib) and lenalidomide is poor; with further treatment, median OS is at best 12 months; without further treatment, OS is dismal: 3 months.6 To that end, a number of next-generation novel agents have recently been approved in the US and in Europe7–10 for the treatment of patients with relapsed and refractory multiple myeloma (RRMM), in whom prior therapies including lenalidomide and bortezomib have failed; these include the immunomodulatory drug (IMiD) pomalidomide, the proteasome inhibitor carfilzomib and the deacetylase inhibitor panobinostat.
In particular, pomalidomide plus dexamethasone is approved both in the US and Europe for the treatment of patients with RRMM who have received ≥2 prior therapies (including lenalidomide/thalidomide + bortezomib) and have progressed on treatment or within 60 days following completion of their last prior therapy.7,10
We will describe the development of pomalidomide as a novel therapy for RRMM and will include discussion of the known mechanism of action, the establishment of the approved dose and schedule (phase I studies), safety and efficacy trials in RRMM (phase II and III trials), and novel pomalidomide-based combinations being investigated. The review will then explore additional recent advances in the treatment of RRMM and the role of pomalidomide in this context. We will conclude with a discussion of the array of populations of patients with RRMM who may benefit from treatment with pomalidomide, including patients with renal impairment, patients with high-risk cytogenetics and the elderly.
Proposed Mechanisms of Action
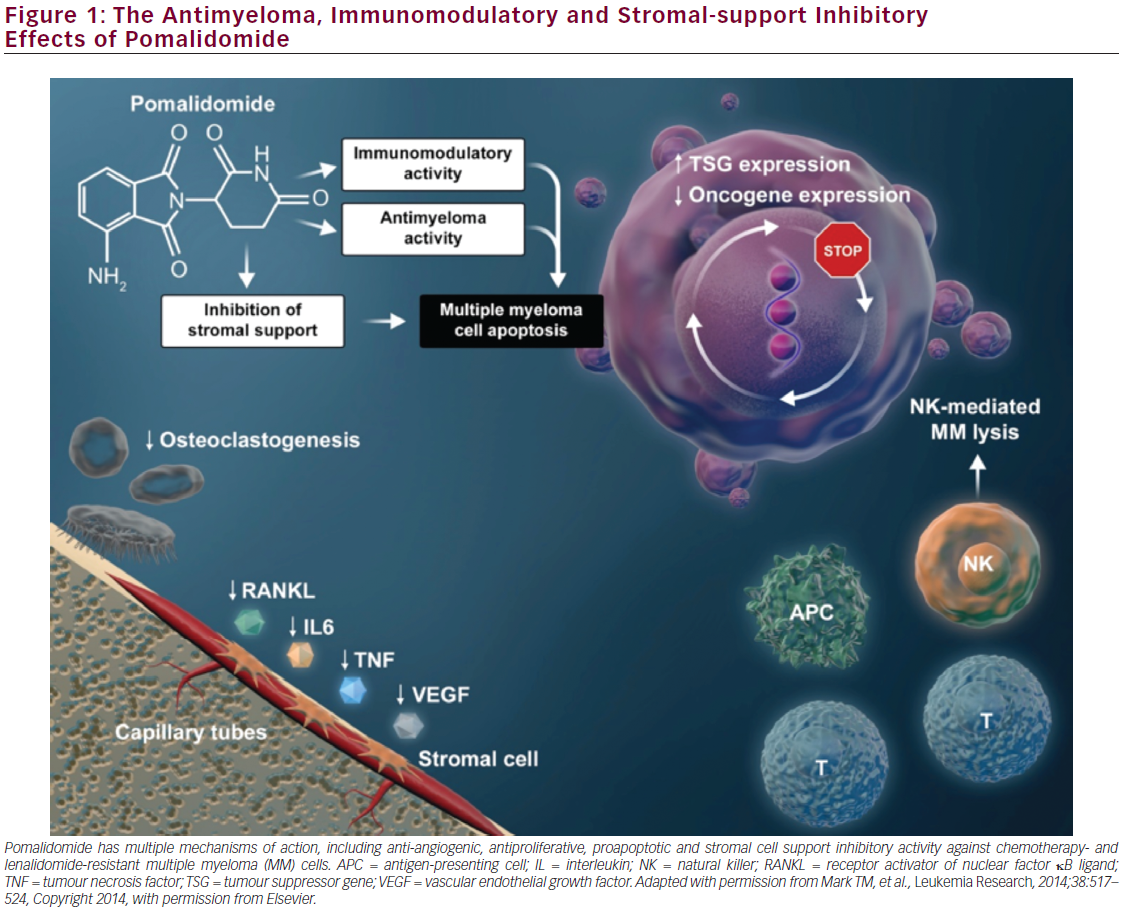
Pomalidomide has potent antimyeloma and immunomodulatory activities as well as inhibitory effects on stroma cells11–17 that can facilitate combination therapy with agents with complementary mechanisms of action, resulting in significant antimyeloma effects and non-overlapping toxicities. The mechanisms of pomalidomide’s pleiotropic antimyeloma effects are complex (see Figure 1). Direct tumoricidal activity is mediated by inhibition of DNA synthesis and proapoptotic signalling.12 Importantly, pomalidomide inhibits proliferation of lenalidomide-resistant MM cell lines18 and has synergistic activity with dexamethasone in both lenalidomide-sensitive and lenalidomide-resistant cells.19 This activity is potentially mediated through suppression of IRF4, a gene critical for myeloma cell growth, as well as suppression of expression of the protooncogene c-Myc to affect up-regulation of the cyclin-dependent kinase (CDK) inhibitor p21WAF-1 and cell cycle arrest.18 Immunomodulatory effects
of pomalidomide include enhancement of T-cell- and natural killer cellmediated immunity.11,20–22
Further immunomodulatory activity of pomalidomide is mediated through inhibition of the production of pro-inflammatory cytokines, including tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6).11 T-cell and TNF-α immunomodulatory activities of pomalidomide and other immunomodulatory agents (thalidomide and lenalidomide) have recently been shown to be regulated by their binding to the ubiquitously expressed E3 ubiquitin ligase protein cereblon to affect T-cell activation.18,23,24 Pomalidomide and lenalidomide promote binding of the transcriptional repressors of IL-2 expression, Ikaros and Aiolos, to cereblon, thereby facilitating the subsequent ubiquitin-mediated proteosomal degradation of these repressors in T-lymphocytes. Hence, pomalidomide-enhanced, and thus cereblon-mediated, degradation of Ikaros and Aiolos promotes T-cell activation through de-repression of IL–2.25-27
Similar to its analogue thalidomide, pomalidomide has antiangiogenic effects, as demonstrated by in vitro angiogenesis assays.28 Pomalidomide also has anti-inflammatory effects that are due, in part, to elevation of IL-10 production and subsequent inhibition of cyclooxygenase-2 expression.29,30
The complex and diverse activities of pomalidomide in both chemotherapy- and lenalidomide-resistant cells not only make it an attractive therapy for patients with refractory disease but also allow for the design of combination therapy regimens that take advantage of nonoverlapping toxicities and complementary mechanisms of action. Such pomalidomide combination regimens have in turn been assessed in trials that have studied such combinations and will be discussed below.
Establishment of Approved Dose and Schedule
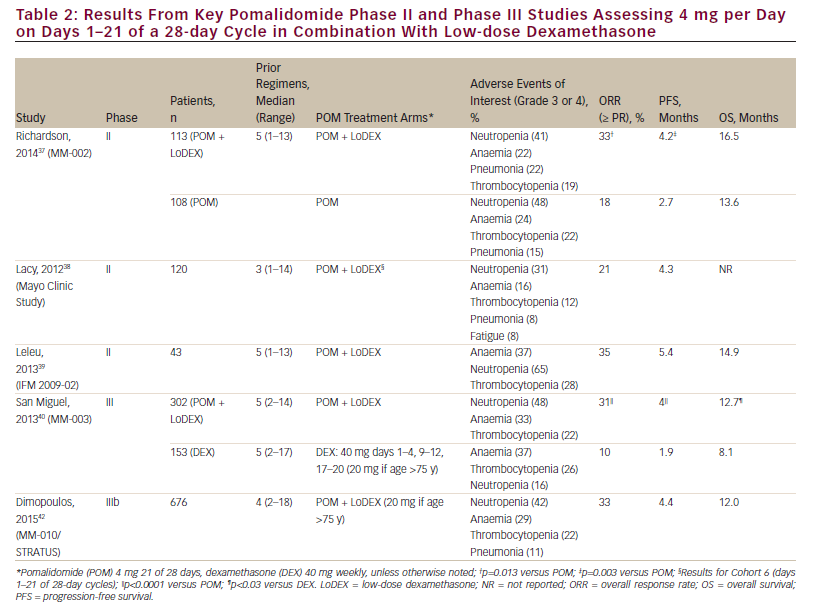
Several early-phase trials evaluated dose-limiting toxicities in order to determine the maximum tolerated dose and the optimal treatment schedule of pomalidomide in patients with RRMM who had previously received multiple lines of treatment, including bortezomib and lenalidomide (see Table 1). Initially, pomalidomide was explored as a single agent before being investigated in combination with dexamethasone.31,32 The approved dose was based on the data from MM-002, which assessed pomalidomide at 2, 3, 4 or 5 mg/day doses in combination with 40 mg weekly dexamethasone.33 The most commonly reported grade 3 or 4 treatment-emergent adverse events in MM-002 were neutropenia, anaemia, thrombocytopenia and fatigue (53 %, 21 %, 18 % and 16 %, respectively). Except for one case of grade 3 fatigue in a patient in the pomalidomide 2 mg cohort, all of the doselimiting toxicities in this study were grade 4 neutropenia and occurred across the 3, 4 and 5 mg dosing levels.33 The maximum tolerated dose and schedule for which pomalidomide has been approved in RRMM is 4 mg per day on days 1–21 of a 28-day cycle in combination with 40 mg weekly dexamethasone, although other doses and schedules were analysed for pomalidomide monotherapy31,32 and in combination with low-dose dexamethasone.33–36
Clinical Trials Aimed at Assessing Safety and Outcomes
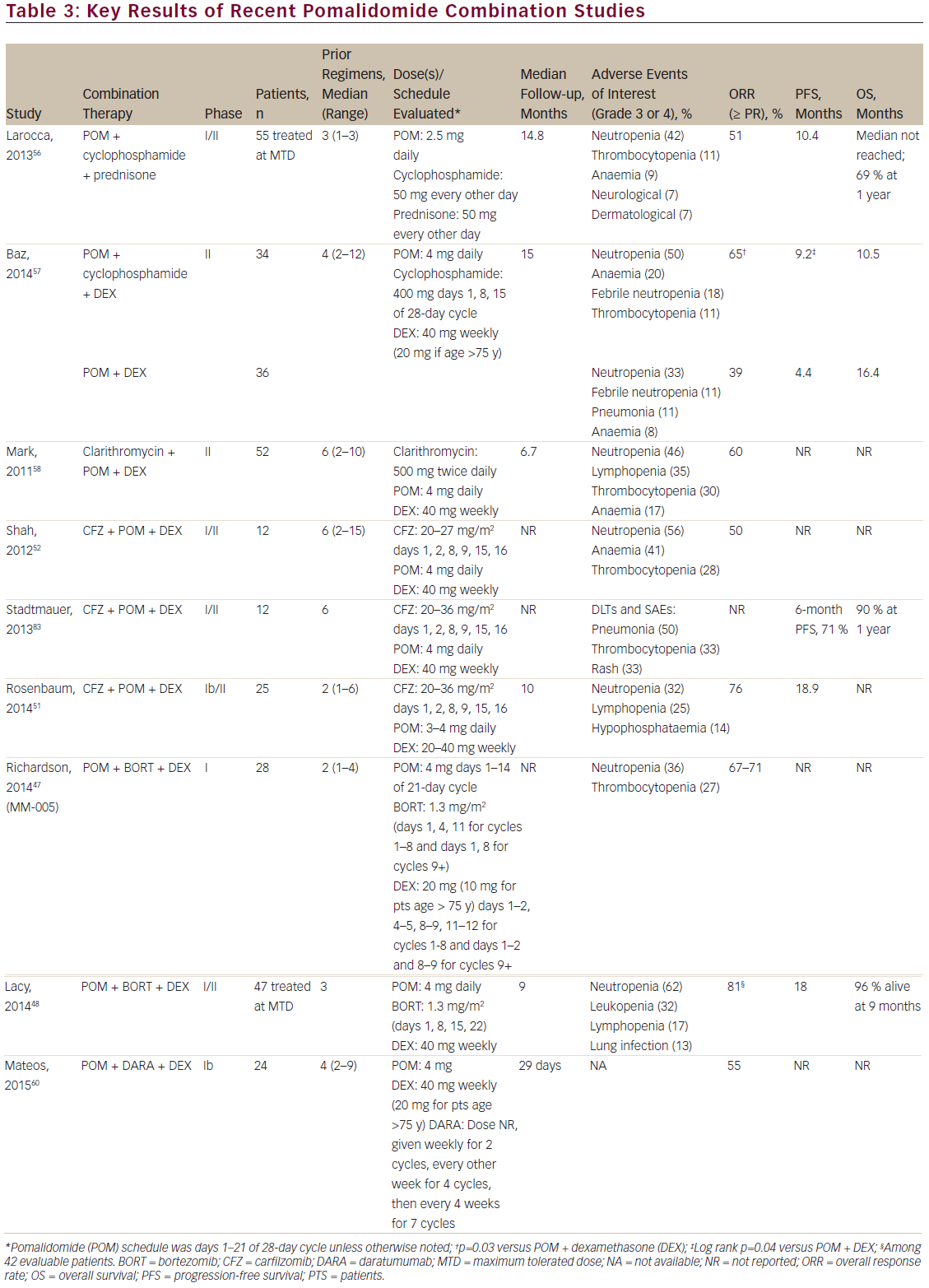
Multiple studies have examined the safety and efficacy of pomalidomide at the approved dose and schedule in combination with low-dose dexamethasone. Table 2 summarises the key results of the MM-002 phase II study as well as the Intergroupe Francophone du Myélome 2009-02 (IFM 2009-02) phase II study, the Mayo phase II study and the MM-003 and STRATUSTM (MM-010) phase III studies,
which all assessed the safety and clinical efficacy of pomalidomide + dexamethasone for the treatment of RRMM. The pivotal phase II portion of MM-002 evaluated pomalidomide alone or in combination with lowdose dexamethasone.37 All patients had to have received ≥2 prior antimyeloma therapies, including ≥2 cycles of lenalidomide and ≥2 cycles of bortezomib, which were given separately or in combination, and had to have relapsed after having achieved at least stable disease for ≥1 cycle of treatment with ≥1 prior regimen. The patients’ RRMM must have progressed during or within 60 days of their completing treatment with the regimen used just prior to study entry (defining relapsed and refractory disease).37 Overall response rate (ORR; ≥ partial response) was significantly higher with pomalidomide + low-dose dexamethasone compared with pomalidomide alone (33 % versus 18 %, p=0.013) with a median follow-up of 14.2 months.37 Progressionfree survival (PFS) was also greater in the pomalidomide + low-dose dexamethasone group (4.2 versus 2.7 months; p=0.003). Importantly, refractoriness to lenalidomide or resistance to both lenalidomide and bortezomib did not affect outcomes with pomalidomide + low-dose dexamethasone. Pomalidomide + low-dose dexamethasone showed significantly greater PFS than pomalidomide alone in the subset of patients with lenalidomide-refractory disease (3.8 versus 2.2 months; p=0.042) and trended towards greater PFS in patients with lenalidomideand bortezomib-refractory disease (3.8 versus 2.0 months; p=0.150).37 The median OS was 16.5 and 13.6 months in the pomalidomide + lowdose dexamethasone and pomalidomide alone groups, respectively.37 This compares favourably with historically reported 9-month survival rates in patients in whom currently approved novel agents have failed.6 The results of the MM-002 study confirmed those of earlier studies in which 4 mg pomalidomide on days 1– 21 of a 28-day cycle + weekly low-dose dexamethasone was assessed for the treatment of RRMM.38,39 Thus, the studies described here demonstrated that there is no cross-resistance between pomalidomide and lenalidomide in patients who had received prior treatment with lenalidomide or lenalidomide + bortezomib.
Further evidence for the effectiveness of pomalidomide + low-dose dexamethasone in patients whose disease was refractory to lenalidomide or both lenalidomide and bortezomib has recently been demonstrated in two phase III studies.40–42 In the multinational MM-003 trial, pomalidomide + low-dose dexamethasone versus high-dose dexamethasone was assessed in 455 patients with refractory MM or RRMM who had failed at least two previous treatments of bortezomib and lenalidomide.40 With a median follow-up of 15.4 months, longer PFS (4.0 versus 1.9 months, hazard ratio [HR]: 0.50; p<0.001) was achieved in patients receiving the combination of pomalidomide + low-dose dexamethasone versus high-dose dexamethasone alone.41 This PFS benefit was also seen in patients with lenalidomide-refractory disease (HR 0.51 [95 % confidence interval (CI), 0.41–0.64]) and lenalidomide- and bortezomib-refractory disease (HR 0.53 [95 % CI 0.42–0.68]). In the final OS analysis, median OS was significantly longer in patients treated with the combination (13.1 versus 8.1 months, HR 0.72; p=0.009).41 Indeed, the OS of 13.1 months in the pomalidomide + low-dose dexamethasone arm compares very favourably with the 9-month survival rates for patients in whom currently approved novel agents have failed.6 The most common grade 3/4 haematological adverse events in the pomalidomide + low-dose dexamethasone and high-dose dexamethasone groups, respectively, were neutropenia (48 % versus 16 %), anaemia (33 % versus 37 %) and thrombocytopenia (22 % versus 26 %).40
The phase III MM-010 study is the largest conducted to date with pomalidomide + low-dose dexamethasone in a heavily pretreated RRMM patient population (median four prior therapies; 80 % refractory to lenalidomide and bortezomib).42 Treatment with pomalidomide + low-dose dexamethasone resulted in an ORR of 33 % with a median PFS and OS of 4.4 and 12.0 months, respectively.42 Importantly, these results were similar to those in the subgroup of patients refractory to lenalidomide or lenalidomide + bortezomib: PFS of 4.4 and 4.2 months, respectively, and OS of 12.0 months for each. The most common grade 3/4 treatment-emergent adverse events were haematological and included neutropenia (48 %), anaemia (31 %) and thrombocytopenia (23 %).42 MM-010 confirmed the findings of MM-002 and MM-003, and demonstrated that the combination of pomalidomide + low-dose dexamethasone should be considered a standard of care for patients with RRMM in whom lenalidomide and bortezomib treatment has failed.
In a pooled analysis of six trials comprising 641 patients treated with pomalidomide + low-dose dexamethasone for lenalidomide and bortezomib dual-refractory RRMM, response rates were found to be consistent, with an ORR of 31 %.43 In both the pooled analysis and MM- 010, the ORR in patients aged >65 years was 32 %.
Novel Combinations with Pomalidomide
The encouraging results of pomalidomide in combination with dexamethasone and its favourable toxicity profile have provided the impetus to explore combination regimens of pomalidomide with other antimyeloma drugs in the relapsed/refractory setting. Since additive toxicities among agents may be avoided or minimised by judicious selection of agents with non-overlapping toxicities and mechanisms of action, unique combinations may result in additive or synergistic antimyeloma effects. Pomalidomide’s manageable toxicity profile could allow longer duration of therapy, thus providing durable responses that may be augmented by the addition of antimyeloma therapies with complementary mechanisms of action to help achieve a deeper response. While the association of depth of treatment response to outcomes is not universal across studies, improvements in the quality of response (depth and durability) across all stages of treatment are associated with better disease control and longer survival.45,46 It should also be considered that exposure of malignant cells to a single agent often results in preferential overactivation of alternate cell survival pathways that could be targeted using other agents with alternative mechanisms of action.45
Table 3 summarises early-phase trials and encouraging results of some pomalidomide-based combination studies in RRMM. For example, since pomalidomide retains activity in patients with lenalidomide- and bortezomib-refractory disease,33,37–40,42 pomalidomide + dexamethasone is therefore a logical backbone for combination therapy in patients with RRMM who are refractory to lenalidomide and/or bortezomib. Similarly, the combination of pomalidomide, bortezomib and dexamethasone is a rational approach to treating patients who are refractory to lenalidomide. Indeed, high responses (approximately 70–80 %) have been seen in early-phase trials assessing this combination in lenalidomide-refractory patients.47,48 Initial results of a phase I study assessing the triplet therapy of pomalidomide (4 mg/day, days 1–21 of a 28-day cycle), bortezomib (1 mg weekly) and low-dose dexamethasone have demonstrated the utility of this regimen in 47 patients with RRMM.48 A large proportion of these patients were high-risk (del[17p], t[4;16] or t[14;20]) by fluorescent in situ hybridisation [FISH] or high-risk gene expression profiling [GEP] signature)5 and heavily pretreated. Response rates were 84 % in 19 high-/intermediate-risk (t[4;14] by FISH, cytogenetic del[13], hypodiploidy or plasma cell labelling index ≥3 %) patients and 86 % in 28 standard-risk patients (all other patients including t[11;14] and t[6;14]). Median PFS was 9.5 months in high-/intermediate-risk patients and 16.3 months in standard-risk patients.48 The most common adverse events (including those grade ≥3) were haematological and included anaemia, thrombocytopenia and neutropenia.48 This combination is being further explored (versus bortezomib + dexamethasone) in the ongoing phase III trial OPTIMISMM [NCT01734928] with an expected enrollment of 782 patients with RRMM.49 The results of this trial are eagerly awaited, as they will help address the question of whether adding pomalidomide to bortezomib and dexamethasone will provide a deep response and extend the duration of response compared with bortezomib and dexamethasone salvage therapy. Similar to the combination of pomalidomide, bortezomib and dexamethasone, the combination of pomalidomide, carfilzomib and dexamethasone has also provided high rates of response, with acceptable safety profiles.50–52 Larger studies are needed to confirm these results and are under way.
The results of the OPTIMISMM trial will likely be considered in the context of results from ENDEAVOR, a phase III trial (n=929) in RRMM in which the carfilzomib + dexamethasone arm had a median PFS nearly twice that of the bortezomib + dexamethasone arm: 18.7 versus 9.4 months.53 However, the significant improvement in PFS is also tempered by an increase in grade ≥3 adverse events of interest in the carfilzomib arm: hypertension (9 % versus 3 %), dyspnoea (preferred term; 5 % versus 2 %), cardiac failure (5 % versus 2 %) and acute renal failure (4 % versus 3 %).53 Indeed, the safety results from ENDEAVOR are not dissimilar to those of the single-agent carfilzomib arm of the FOCUS trial.54 Overall, caution may be warranted when using carfilzomib in patients with cardiac comorbidities or who have received prior therapy with cardiotoxic agents.55
Combinations of pomalidomide and cytotoxic agents have been evaluated in early-phase trials in patients with RRMM. The combination of pomalidomide, cyclophosphamide and prednisone was evaluated in a phase I/II trial, with a reported ORR of 51 %.56 The combination of pomalidomide, cyclophosphamide and dexamethasone was evaluated in a phase II study and demonstrated superior ORR (65 %) and PFS (median 9.2 months) versus pomalidomide plus dexamethasone (ORR of 39 % and median PFS of 4.4 months).57 The combination of pomalidomide, dexamethasone and clarithromycin was evaluated in a phase II study, with a reported ORR of 60 %.58 Additionally, reduced-dose pomalidomide, dexamethasone and pegylated liposomal doxorubicin has been evaluated in a phase I/II trial in lenalidomide-refractory patients and demonstrated an ORR of 43 % and a good tolerability profile.59
Pomalidomide is also being evaluated in combination with the anti-CD38 monoclonal antibodies daratumumab and isatuximab (SAR650984). The
safety and tolerability of daratumumab combined with pomalidomide and dexamethasone was investigated in 24 patients in a recent phase Ib study.60 Patients who had received ≥2 consecutive cycles of bortezomib and lenalidomide and had refractory or relapsed and refractory multiple myeloma received the approved dose of pomalidomide and dexamethasone and weekly daratumumab (for cycles 1 and 2, then every other week for cycles 3–6, and every 4 weeks for seven cycles). The most common adverse events were haematological and were likely related to pomalidomide. The ORR was 55 %, with a median time to first response of 31 days (range, 29–57 days).60
Although results are not yet available for the combination of isatuximab with pomalidomide, some early data have emerged for isatuximab with lenalidomide. A phase Ib study assessing lenalidomide, isatuximab, and dexamethasone in a heavily pretreated RRMM population demonstrated that this combination was well tolerated and yielded rapid and durable responses: the median time to first response was 4.2 weeks and the duration of response was 23.1 weeks.61 With a median follow-up of 6.0 months, median PFS was 6.2 months.61 Importantly, in addition to the responses being evident following the first cycle of therapy and deepening thereafter, the ORR was 63 % in patients who were refractory to their last regimen containing lenalidomide.61 A phase Ib study is currently recruiting and will assess the safety, pharmacokinetics, duration of response, and preliminary efficacy of pomalidomide, isatuximab and dexamethasone in patients with RRMM (NCT02283775).62
Special Populations that May Benefit from Pomalidomide Therapy
RRMM may be particularly challenging to treat in patients with comorbidities or other factors that may limit dose intensity or duration of therapy. When selecting a therapeutic regimen, physicians must consider associated toxicities and the fitness of patients who receive these therapies. Patient populations for which this approach may be especially important include the elderly, patients with renal disease and patients with high-risk cytogenetics.
Elderly
As the general population ages, treatment of the elderly RRMM patient must be increasingly considered. Although not always, elderly patients are often ineligible for high-dose chemotherapy and autologous stem cell transplant. Effective therapies that are associated with limited toxicities are therefore needed. Pomalidomide at 4 mg has been shown to be an appropriate starting dose in patients with RRMM regardless of age.44 In a recent subanalysis of the MM-010 study assessing 4 mg pomalidomide + low-dose dexamethasone (40 mg weekly ≤75 years, 20 mg weekly >75 years), Palumbo et al.,44 examined outcomes by age (≤65 versus >65 years, and ≤70 versus >70 years). ORR was consistent for patients aged ≤65 (34 %), >65 (32 %), ≤70 (32 %) and >70 (34 %) years.44 Median PFS was also similar across all age groups (PFS range, 4.2–4.7 months).44 Similar toxicity rates were noted across the age groups. Grade 3/4 adverse effects included neutropenia (47–48 %), anaemia (29–31 %), thrombocytopenia (18–25 %) and pneumonia (10–13 %).44 These results support those of previous subanalyses of the MM-002 and MM-003 trials assessing the efficacy and safety of pomalidomide by age.63,64 Pomalidomide and low-dose dexamethasone has therefore been shown to be an effective treatment option for RRMM regardless of age.
Renal Impairment
Renal impairment is a common comorbidity in patients with MM, with >20 % of patients ultimately experiencing renal failure.65 Pomalidomide is extensively metabolised prior to excretion, but since 10 % is excreted unchanged in the urine,66 clinically relevant nephrotoxicity is limited. This is similar to thalidomide, but distinct from lenalidomide, which excretes >80 % of the parent compound in urine.67,68 Pharmacokinetic data indicate similar mean dose-normalised exposure of pomalidomide in patients with RRMM with severe renal impairment (creatinine clearance [CrCl] <30 mL/minute; including patients requiring and not requiring dialysis) and those with normal renal function (CrCl ≥90 mL/minute) or mild renal impairment (CrCl ≥60 to <90 mL/minute) at the approved 4 mg dose.69 Pomalidomide clearance is not significantly affected by renal function (as measured by CrCl or renal impairment).70 Similarly, in a study of clarithromycin + pomalidomide + dexamethasone, baseline renal function as well as hepatic function were found not to be predictive of dose reduction.71 In renally impaired patients, pomalidomide + lowdose dexamethasone has an acceptable safety and efficacy profile comparable to that observed in patients without renal impairment. In several studies, response to pomalidomide + low-dose dexamethasone and tolerability were consistent across renal function subgroups, with few discontinuations due to adverse effects.71–74 These results were most recently supported by an analysis of patients with RRMM and renal impairment in the MM-010 STRATUS trial.75 In patients with moderate renal impairment (CrCl ≥30 to <60 mL/minute), treatment with pomalidomide + low-dose dexamethasone had a manageable safety profile and was efficacious. Tolerability was similar in patients with or without moderate renal impairment, which was consistent with results from the MM-002 and MM-003 trials.37,40 Despite differences in patients’ renal function, ORRs were consistent between groups. Although there was a slightly longer median PFS in patients without moderate renal impairment, this did not meet statistical significance (p=0.1644).75 However, data in patients with severe renal impairment (CrCl <30 mL/minute) remain limited. Use of pomalidomide should be avoided in patients with a serum creatinine level >3.0 mg/dL.7
High-risk Cytogenetics
Patients with MM and high-risk cytogenetics (such as del[17p] and t[4;14]) have an early relapse rate and shorter survival.76,77 However, in patients with RRMM receiving salvage therapy with pomalidomide + low-dose dexamethasone, high-risk cytogenetics do not appear to affect outcomes in non-heavily pretreated patients76 or heavily pretreated patients.78–80 This combination is therefore effective and has been shown in several studies to be well tolerated in patients with RRMM and adverse cytogenetics, particularly those with del(17p), a patient population that often rapidly becomes refractory to therapy and has a poor prognosis.77,81 However, the relationship between specific cytogenetic abnormalities, risk and response to therapy is less defined. Indeed, some studies have shown differential effects of specific abnormalities,39 and additional studies are needed in this area.
Conclusions
Pomalidomide is a distinct immunomodulatory agent that, in combination with low-dose dexamethasone, has significant activity in patients with RRMM in whom prior lenalidomide and bortezomib have failed, a patient population for whom there are limited treatment options and that constitutes an important unmet medical need. Importantly, pomalidomide’s activity is maintained in difficult-to-treat patient subgroups, including the elderly, patients with adverse cytogenetics and those with renal impairment. Moreover, its favourable tolerability profile and manageable toxicity make it additionally attractive as a therapeutic option, especially in combination and given the active dose range seen with its use, which in turn allows a tailored approach to its use.82 In addition, the pleiotropic mechanism of action of pomalidomide, including direct tumoricidal, immunomodulatory, anti-angiogenic and anti-inflammatory activity, indicates that pomalidomide can be used in combination with agents that have complementary mechanisms of action to provide a greater antimyeloma effect than single-agent therapy or previous combination therapies. Indeed, pomalidomide in combination with proteasome inhibitors and traditional chemotherapeutic agents in doublet or triplet regimens is providing high rates of response that are durable. These studies and other pomalidomide-based combinations with various novel therapies are especially important new treatment options for patients with RRMM, for whom effective new therapies are urgently required.














