Lung cancer is the most common non-cutaneous cancer globally, and the foremost cause of cancer mortality.1 Non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancer.2 Forty to fifty percent of patients diagnosed with NSCLC have disease which is incurable at presentation, due to pleural or pericardial effusion, or metastatic (distant) spread;2,3 the latter is classified as stage IV. The treatment for widely metastatic stage IV NSCLC is typically palliative-intent systemic therapy and/or radiotherapy (RT), with goals of increasing survival, improving quality of life (QOL), and controlling symptoms. Five-year overall survival (OS) for stage IV NSCLC is 1%.4
Just over 20 years ago, Hellman and Weichselbaum coined the term ‘oligometastases’ (OM), describing a transitional state of metastatic disease burden, intermediate between relatively localized, and widely distributed.5 The exact definition of OM continues to be debated, frequently comprising five or fewer lesions outside of the primary tumor and regional lymph nodes (LNs),6 often additionally limited to one or two anatomic subsites. Approximately 7% of patients with metastatic NSCLC, in fact, have a solitary metastasis.7 Reported 5-year OS for oligometastatic NSCLC ranges from 14–48%, depending on site(s) of OM and intrathoracic stage.6
Ablative treatment, if delivered to sites of OM before the disease acquires the genetic alterations which confer widespread metastatic potential, could theoretically: increase the interval over which the disease is controlled; decrease metastatic load and subsequent seeding of additional locations; delay progression, at least locally; postpone initiation or switch of systemic therapy, avoiding the resulting toxicity, cost and detrimental impact on QOL; and possibly increase OS.5,8–10 This scenario may be especially relevant to epidermal growth factor receptor (EGFR) mutant, or anaplastic lymphoma kinase (ALK) rearrangement patients who experience oligoprogression during therapy with a targeted agent, in whom radical local therapy may allow continuation of the same drug.11
The adrenal gland is one of the common sites of metastases from NSCLC, with 30–60% harboring such lesions at autopsy.3 Four to ten percent of patients with otherwise operable NSCLC will have a unilateral adrenal mass;12–17 up to half of these are malignant (0.4–3.5%).18–22 In this highly selected subset of patients, there may be a window when local therapy to all known metastases, including an adrenal metastasis (AM), confers benefits over a palliative approach.10,23 The objective of this review was to analyze the literature describing aggressive treatment of AM (solitary or as a component of an oligometastatic presentation) secondary to NSCLC.
Methods
A literature search was undertaken including keywords “non-small cell lung cancer”, “treatment”, “radiotherapy”, “surgery”, “neoplasm metastasis/” or “oligometastases”, and “adrenal” (Appendix 1). The search was limited to English language publications (1997–2017), and pathologic confirmation of NSCLC primary was preferred. Studies which included adrenal OM from different primary sites and/or OM from different locations were included if outcomes relevant to our objective could be extracted. Those comparing the results of aggressive local treatment to palliative treatment were eligible. Articles were excluded if no NSCLC or adrenal OM-specific clinical outcomes were described, or case reports describing fewer than four patients, to minimize publication and reporting bias (Appendix 2).
Results
Search results
Eighty-four articles initially identified underwent evaluation and review of reference lists for further potential inclusions. Twenty-nine reporting treatment outcomes after either surgical resection (n=23, published 1990–2015, 464 patients) or RT (n=6, published 2008–2014, 57 patients) met eligibility criteria. Surgery studies included one phase II single arm trial and 22 retrospective reviews (two multicenter; Tables 1–3). RT studies included one phase II single arm, four retrospective reviews, and one not specified (Tables 4–5). There are no published studies directly comparing these two modalities, but four compared outcomes after surgery to contemporaneous cohorts treated with palliative chemotherapy. Average sample size was 20.2 (range 4–148) for surgery series, and 9.3 (range 4–18) for RT publications.
Surgical resection
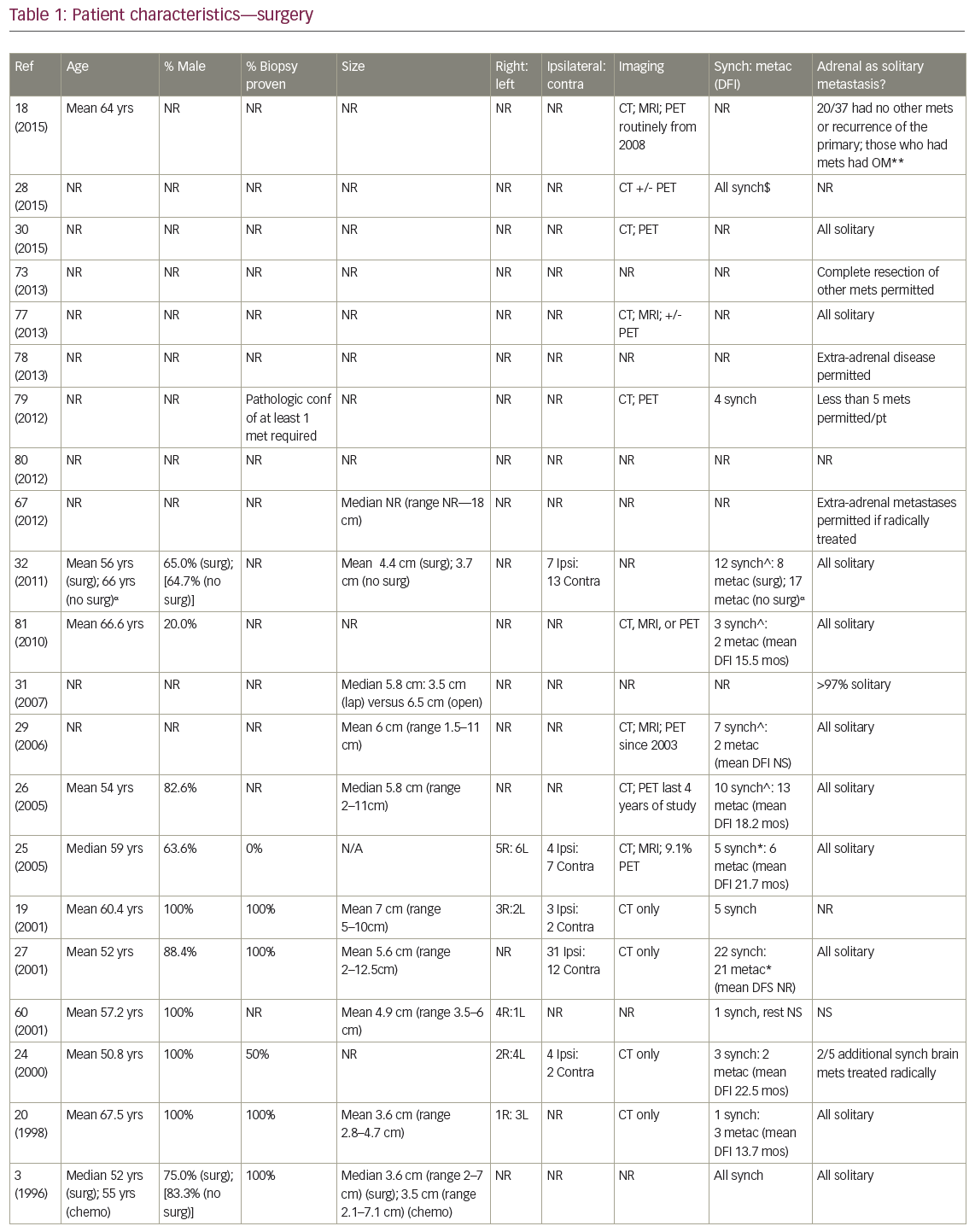
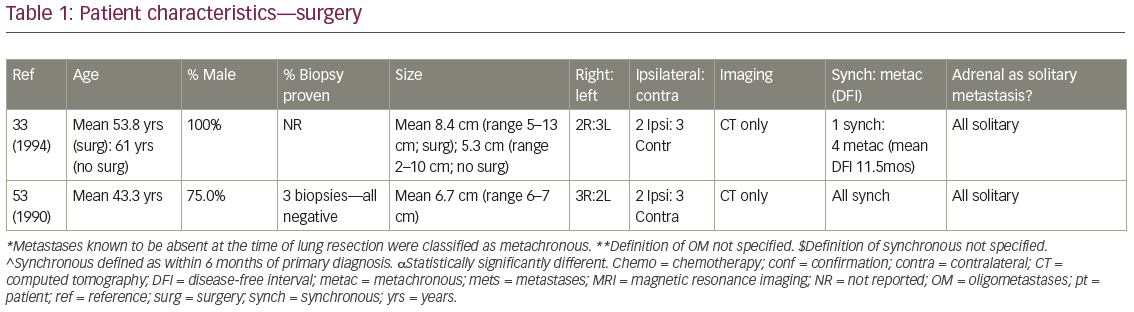
The mean age for patients undergoing adrenalectomy ranged from 43.3–67.5 years (Table 1). With one exception, series were predominantly male. Twelve of the 23 studies required the AM to be the only site of distant spread, while three others allowed extra-adrenal disease if it could also be approached radically, such as resection +/- postoperative RT for brain metastases. Many groups did not specify intrathoracic T stage, and nodal stage varied: some groups excluded LN positive patients altogether,20,24 while the proportion of N2 disease in series where it was permitted varied from 14.3–70.8%.21,25–26 Pathologic confirmation of AM was more commonly performed in early publications, while positron emission tomography (PET) staging became routine later. Mean/median AM sizes ranged from 3.5–8.4 cm, with the largest reported resected 18 cm. Where data were available, on average 49% arose from the right, and 50% from the side ipsilateral to the primary. An average of 64.2% patients presented with synchronous AM, most commonly defined as discovered within 6 months of NSCLC diagnosis.
Five studies described open adrenalectomies, five described laparoscopic, five reviewed patients undergoing both, and the remainder did not specify (Table 1). Regional lymphadenectomy was not routine, except when extraglandular extension was seen.27 Sequencing of surgical resection of the primary and AM varied for synchronous presentations. In Mercier et al.’s series, all patients had the primary removed before adrenalectomy,26 while Bretcha-Boix et al. performed adrenalectomy before lung resection in two-thirds of cases.24 In Porte et al., 12 of the 22 patients (54.5%) were resected simultaneously at the time of lung resection, six of the 22 (27.3%) after lung resection, and four of 22 (18.2%) before.27
Nine studies described receipt of neoadjuvant or adjuvant systemic therapy (or both), while a crude average of 16.1% of patients (range 9.1–20%; n=5 studies) received adjuvant adrenal bed RT. For example, in Mercier’s series, while ‘no particular criteria’ were used by which to recommend adjuvant treatment, nine out of 23 patients – all with negative surgical margins – received a mean of 45 Gy postoperative RT.26
Median survival
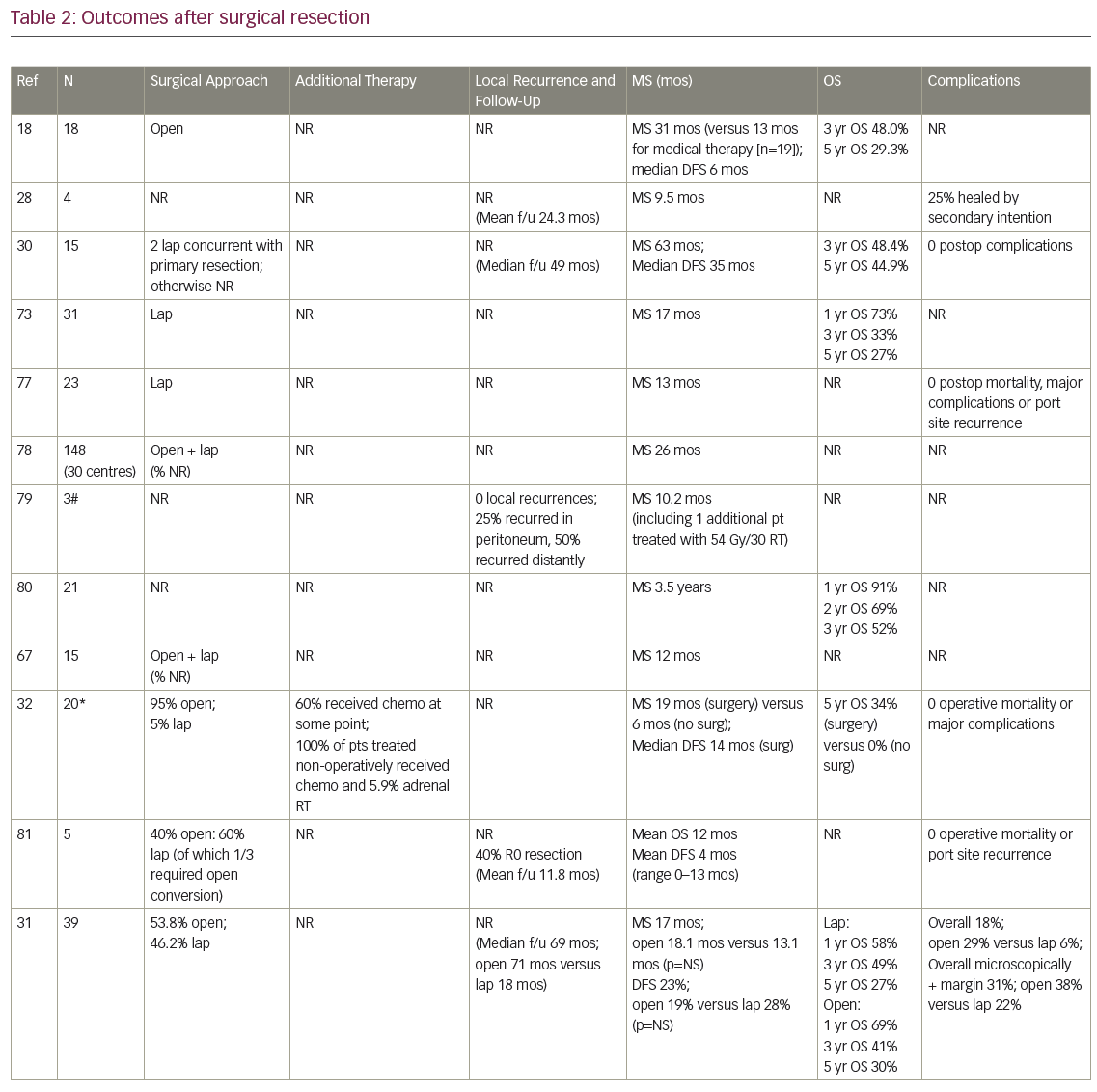
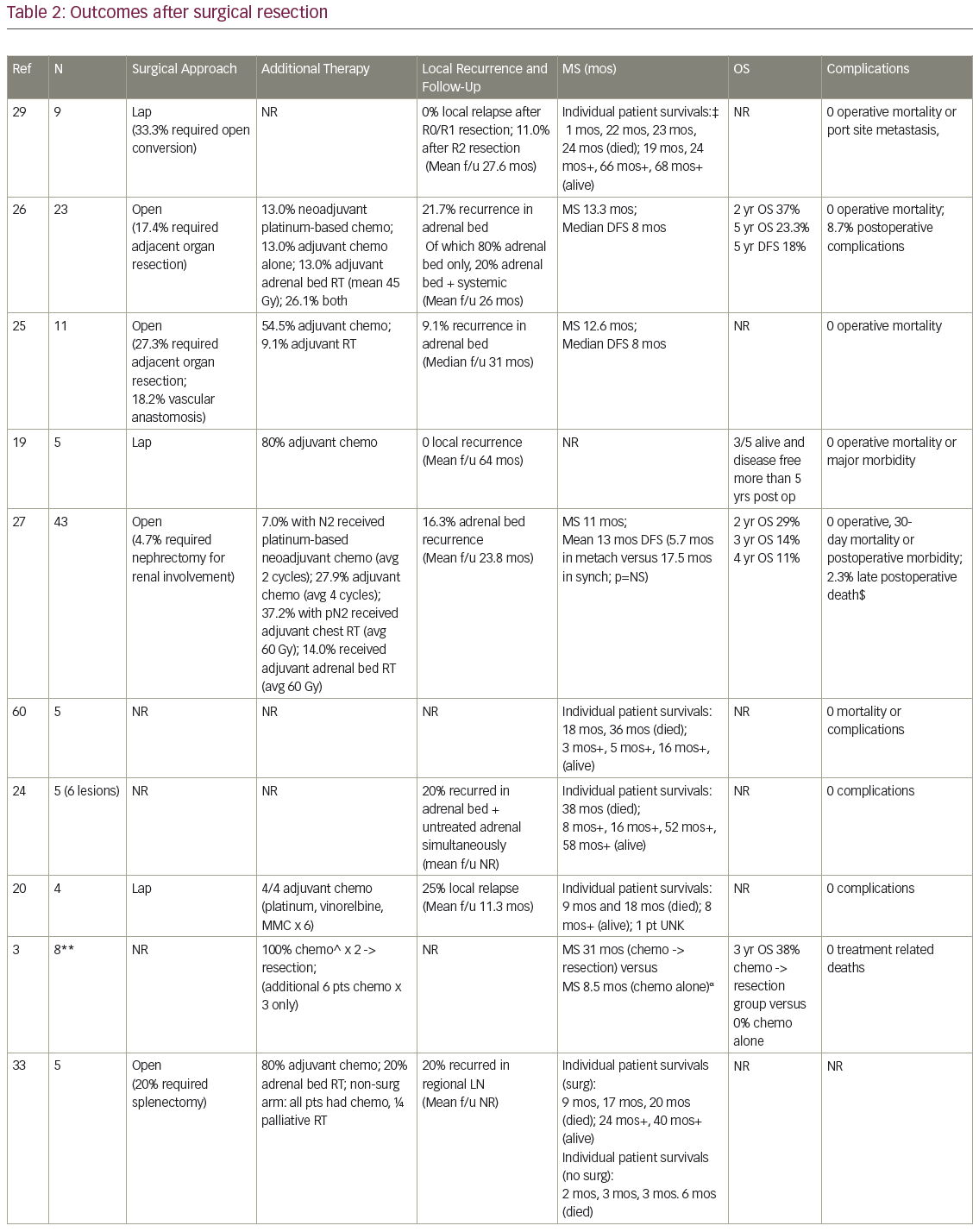
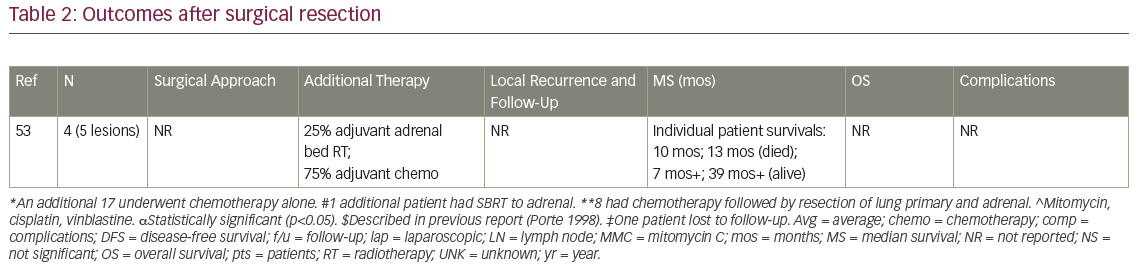
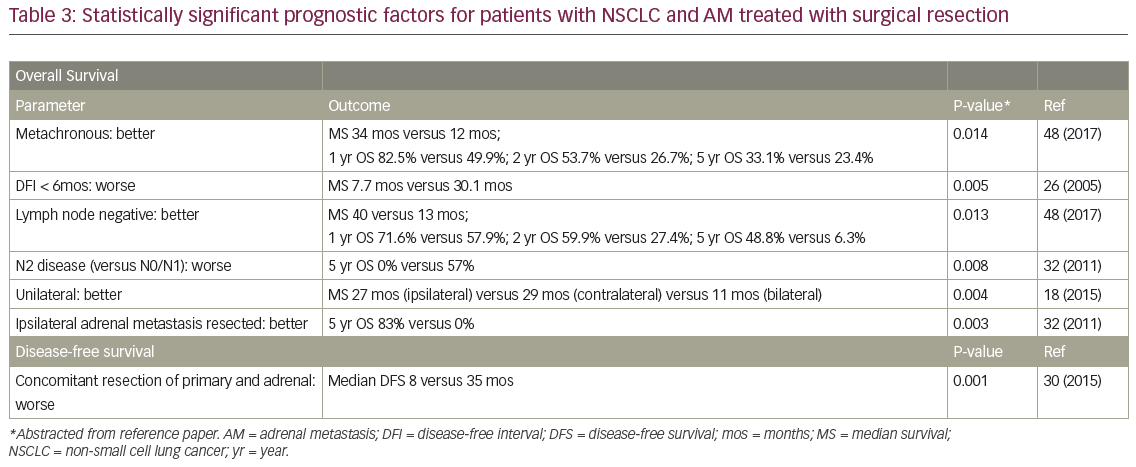
MS after surgical resection ranged from 9.5–63 months (n=16 studies), and median disease-free survival ranged from 4–35 months (n=8; Table 2). Reported 1-year OS ranged from 63.5–91% (n=3), 2-year OS 29–69% (n=3), 3-year OS 14–52% (n=7), and 5-year OS 23–45% (n=6). Plönes et al. found significantly different median survival (MS) for NSCLC patients by location of OM: 23.4 months for soft tissue metastases, 16.7 months for brain, 9.5 months for AM, and 4.3 months for bone metastases (p<0.005).28 Local relapse (LR) occurred on average in 14% (n=8; range 0–25%), with one additional study describing no LR after R0/R1 resections, but 11% local progression after an R2 resection.29 LRs have been associated with capsular invasion26 and intraoperative tumor rupture,30 but not particularly impacted by postoperative RT.26 Surgical complications and postoperative mortality are uncommonly described (Table 2). Table 3 outlines statistically significant prognostic factors for OS and disease-free survival abstracted from surgical series.
Strong’s institutional series—the only one comparing open (n=63) to laparoscopic (n=31) procedures—found no difference in OS, incidence of microscopically positive margins (average 25.5%) or local recurrence (18% overall).31
Surgery versus palliative chemotherapy
In the four studies comparing (predominantly open) adrenalectomy to palliative chemotherapy, treatment decisions were made according to patient/physician preference, often taking into account intrathoracic stage and performance status (PS). Surgery patients tended to be younger, again predominantly male, with smaller AM than those who did not have surgery. Raz et al.’s surgery patients additionally tended to have a better PS, less mediastinal LN involvement (approximately 60% versus 90%) and more metachronous lesions (100% versus 40%).32 MS for patients treated surgically (n=3 studies) was 19 months, 31 months, and 31 months, compared to 6 months, 8.5 months, and 13 months, respectively, after non-surgical treatment. In Luketich et al., 3-year OS was 38% (surgery) versus 0% (non-surgery),3 and in Raz et al., 5-year OS was 34% (surgery) versus 0% (surgery).32 Higashiyama’s publication had two long term survivors (>24 months) after surgery, compared to a mean survival of 3.5 months (n=4 patients) after palliative non-surgical therapy.33
Radiation therapy
The proximity of the adrenals to radiosensitive structures such as the kidney, stomach, intestine, and spinal cord, historically limited the use of RT in this area due to the risk of normal tissue toxicity.34 However, stereotactic body radiation therapy (SBRT) uses multiple, exceedingly precise, external beams delivering a limited number of high dose treatments, utilizing stereotactic fiducial markers and image guidance to improve accuracy.34–36 Large daily doses can be given safely because the treated volumes are usually small with narrow margins, patient positioning is highly reproducible, multiple non-uniform intensity beams each contribute a small dose along individual paths,11,34–35 and four dimensional computed tomography (CT) simulation (treatment planning) scans provide information about the movement of both the target and normal structures.34 SBRT is typically administered over 5–10 treatments on consecutive or alternate days.34
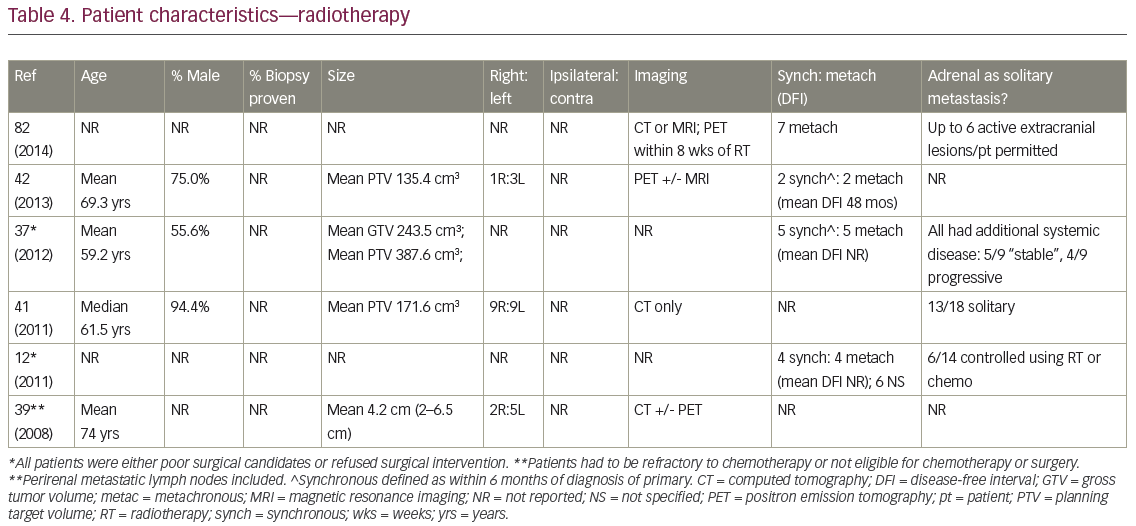
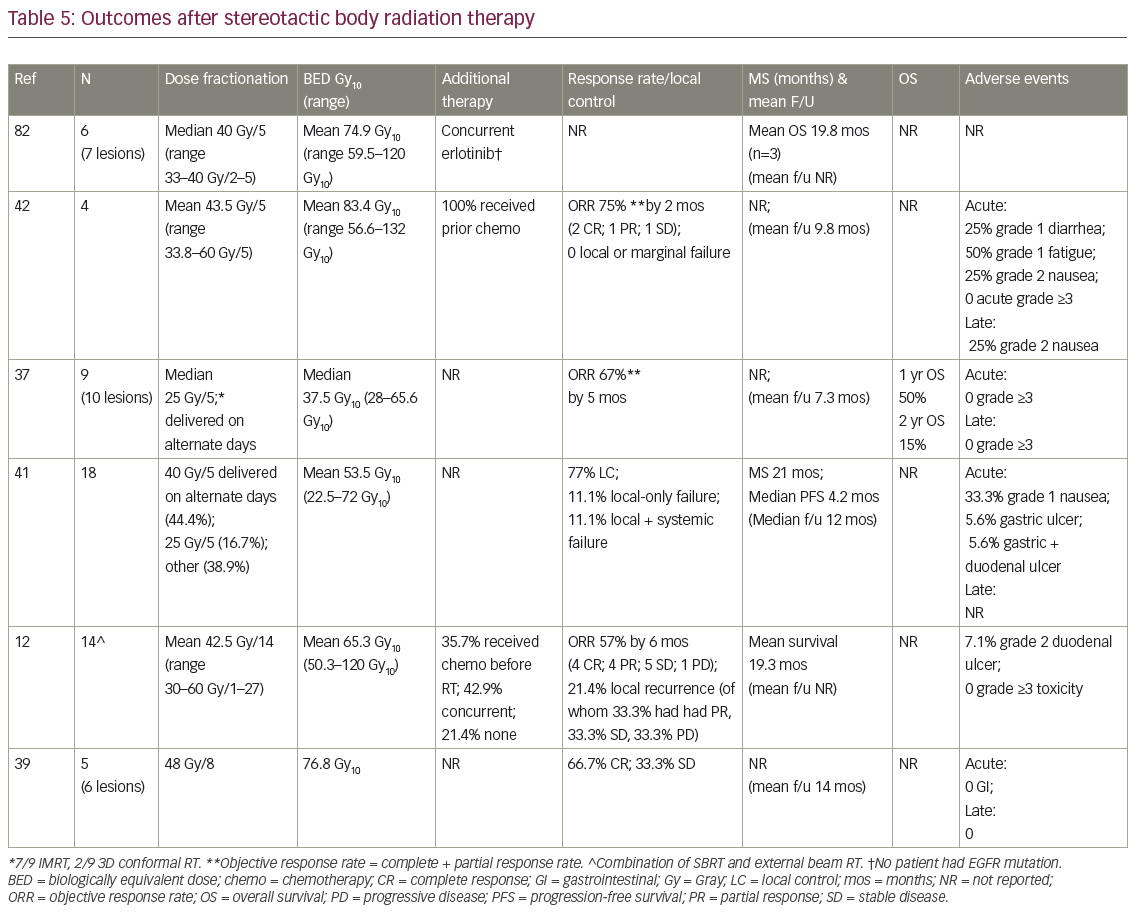
In eligible publications, mean age ranged from 59–74 years (Table 4). As with surgery, patients were >50% male (Table 4). Four studies allowed extra-adrenal disease, of which two included patients with uncontrolled or progressing metastases.12,37 Pathologic confirmation of AM was not reported, while two studies appeared to require PET. In three studies, patients were offered RT only if they were poor surgical candidates, declined surgery, or were refractory to, or declined, chemotherapy. Where data were available, approximately three-quarters of treated AM arose from the right, and on average 37.5% presented synchronously (n=four studies). Patients in three of the studies received systemic therapy before, after, or concurrent with RT (Table 5). However, cytotoxic systemic therapy was commonly held for 2–4 weeks23,38 before SBRT and restarted up to 8 weeks after its completion.38
Simulation
In Katoh et al.’s treatment-planning study, no statistically significant difference in average AM motion in three dimensions was seen between the supine and prone positions.39 There were also no differences in the minimum distances of implanted fiducial markers to the stomach or duodenum. As such, the supine position became standard as it is more comfortable for the patient.39 All patients in modern series had 4D CT simulation scans to enable visualization of the maximum movement of the lesion in relation to normal structures.37 Oral and intravenous contrast were often employed.38 CT-simulation-derived 3D datasets contained images in three different breathing phases: non-contrast free-breathing, contrast-enhanced end-expiration, and contrast-enhanced end-inspiration.40 Simulation and treatment were performed with an empty stomach (nil by mouth [NPO] 3–4 hours) for more reproducible anatomy.39,41 Baseline diagnostic scans (CT, magnetic resonance imaging [MRI], PET) were fused with the simulation scan for contouring.10,42,43
Respiratory motion management
Abdominal compression was often utilized to minimize respiratory excursion; however, approximately 20–40% of patients could not tolerate it due to body habitus, pain or respiratory function.37,41 Respiratory gating or end-expiratory breath hold were employed as other approaches, often implemented if organ motion was greater than a predetermined threshold, such as 5 mm. Implanted fiducials, used by Katoh et al. for real-time tumor tracking, were not associated with complications or migration during treatment.39 When a fiducial marker moved to within 2 mm of the planned coordinates relative to the isocenter, beam-on was triggered.39
Target volumes
The gross tumor volume (GTV) was contoured on both the end-expiration and end-inspiration scans, and combined into an internal target volume (ITV), which encompassed the maximal movement of the lesion in three dimensions.42 The ITV was copied onto the free-breathing sequence, and then geometrically expanded by 3–5 mm.37–39,42,43 Planning target volume (PTV) expansion could be differential – such as 5 mm in the transverse and 10 mm in the craniocaudal dimensions – given that amplitude of movement is often smaller laterally39,41. PTV was reduced or absent near normal structures such as the stomach or duodenum.39,44 Additional margin for subclinical disease (clinical target volume [CTV]) was often 0 mm,37,43 but previous reports described utilization of 2 mm,41 3 mm,38,39 or 5–10 mm.12 Mean PTV size (n=three studies reporting) ranged from 135.4–387.6 cm3, with one additional study describing a mean lesion size of 4.2 cm. Katoh et al. treated patients with perirenal metastatic LN;39 although inclusion of involved regional LN would presently be considered palliative RT.34
Position verification
As the adrenal gland (and therefore neighboring structures) shifts between fractions if the lesion rapidly shrinks, monitoring with cone beam CT enabled early adaptive re-planning, and therefore minimized normal tissue toxicity.37 In two studies, all patients had at least one cone beam CT.37,39 Orthogonal kilovoltage images were used if fiducials were implanted. Any shift greater than 0.3 cm required a second image acquisition to verify position.43
Dose
Total doses ranged from 25–60 Gy, delivered over 1–27 fractions. While biologically equivalent dose (BED) is an unconfirmed method of converting different SBRT dose schedules,35,45 it was utilized for ease of comparison (Table 5). Crude mean BED was 65.2 Gy10 (range 37.5–83.4 Gy10), roughly equivalent to an SBRT schedule of 37.5 Gy/5 fractions.
Outcomes
Complete response (CR) was reported as 28.6%, 50%, and 66.7% (n=3 studies with data); partial response (PR) was 25% and 28.6% (n=2), and stable disease (SD) was 25%, 33.3%, and 35.7% (n=3). Objective response rate (ORR; CR+PR) was 75% 2 months after treatment completion,42 67% at 5 months,37 and 57% at 6 months.12 Crude average local recurrence was 14.5% after RT. Survival was actually uncommonly reported, with mean survivals of 19–20 months and MS of 21 months. Only one study described 1- and 2-year OS (Table 5). There was no reported acute or late grade >3 treatment-related toxicity, and no RT-induced adrenal or renal insufficiency.
Discussion
Traditionally, metastatic NSCLC has been addressed by platinum-based cytotoxic systemic therapy with response rates of 20–30%, and MS of approximately 8 months.46,47 Historically, the MS of patients with an AM, in whom it and the primary are left in situ, is 9 months.22 Five-year OS is essentially zero in the absence of an actionable molecular mutation.4
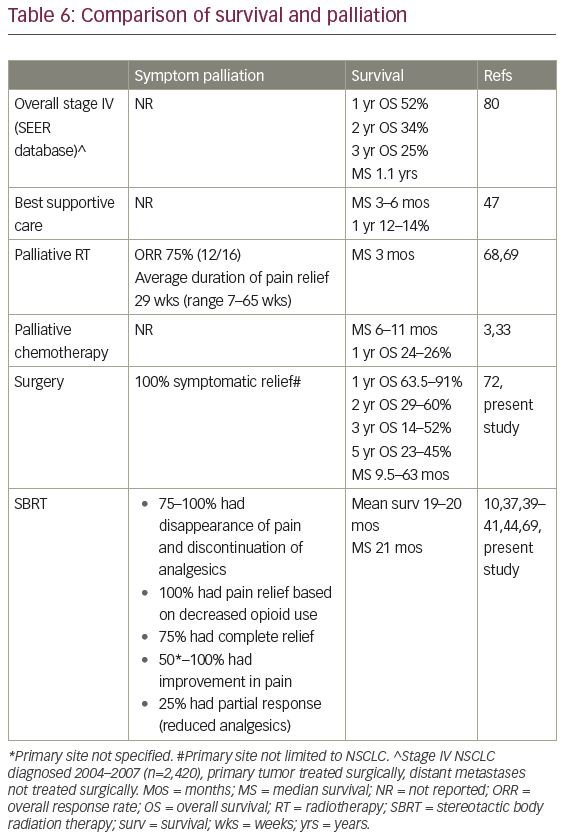
There is no randomized evidence supporting the use of any AM-directed therapy, and no prospective studies directly comparing surgery and SBRT. Limitations of the available data are excellently summarized by Gunjur et al., authors of one of four previous systematic reviews.35 Three of these four papers were restricted to surgical results in lung cancer,48–50 with Gunjur and colleagues analyzing surgery (n=30 studies) and ablative RT (n=9) for adrenal OM from all primary tumor sites.35 Gunjur et al. reported a weighted 2-year OS of 46% for adrenalectomy versus 19% for SBRT, and 2-year local control (LC) of 84% versus 63% respectively.35 Two-year OS after surgery in Gao et al.’s pooled analysis was 40.5%, and 5-year OS was 28.2% (n=98 patients).48 Tanvetyanon et al. described 25% 5-year OS (n=10 studies; 114 patients),49 while Beitler et al. reported approximately one-third of patients as five-year survivors.50 Table 6 contrasts our results with historical survival and symptom palliation rates for widely metastatic stage IV NSCLC.
The better than expected outcomes after treatment of OM may reflect selection of patients with less bulky, slow-growing tumors, good PS, and low overall burden of disease, rather than the effects of aggressive treatment (Table 7).23,51 The relatively poor results of RT in comparison with surgery may also be attributed to differences in patient selection.44 In the surgical literature, the majority of patients have metastases confined to the adrenal or have extra-adrenal OM which have also been aggressively treated; have usually had their primary disease resected; must be technically resectable; and medically operable.44 For example, in Raz et al., all patients managed non-operatively for their, apparently solitary, AM had also been managed non-operatively in the lung.32 Case mix in terms of potential prognostic factors, such as intrathoracic (especially nodal) stage, treatment and status of primary disease, disease-free interval (DFI), PS, expected lifespan, and extra-adrenal metastatic load, are also dissimilar between patients treated with RT and surgery. In terms of treatment, some surgical studies included patients with microscopic margin positivity and/or gross residual disease (R2), while these were excluded by others. RT dose-fractionation schedule varied significantly, with adjuvant and neoadjuvant therapy only briefly described. Early RT is not technologically equivalent to modern treatment; for example, neither cone beam CT nor 4D simulation scans were utilized in early publications. Follow-up and imaging protocols vary widely. Adverse event reporting, especially the late effects of RT, is confounded by competing risks of local progression, distant metastases, death and comorbidity, as well as by subsequent therapy. There are no available QOL data.
Work-up and patient selection
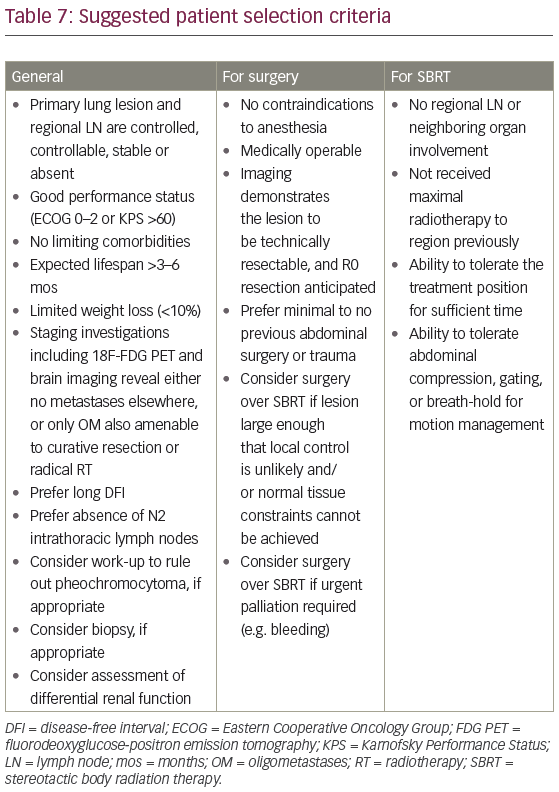
Between 2 and 5% of the population has a benign adrenal adenoma.52,53 Other differential diagnoses include cyst, myelolipoma, pheochromocytoma, and hematoma.52 Imaging features that have been used to characterize metastases include size, growth rate, irregular contour, delayed washout of contrast, associated LN, local invasion/obliteration of fat planes, inferior vena cava (IVC) thrombus and heterogeneity.24,49,54,55 However, CT scans can be falsely negative or underestimate lesion size when metastatic lesions do not alter the macroscopic structure of the gland.56,57 Serum and urine studies,20,54 along with MRI,18,21 should be considered to rule out pheochromocytoma (Table 7). Imaging should ideally have been performed within 2 months of therapy.38
Biopsy of an adrenal mass in this setting remains controversial. It has been proposed as mandatory by some,20,21,24,50,53 especially in a patient with potentially curable intrathoracic disease.57 However, in many publications, only a minority of patients had histological confirmation prior to definitive therapy.10 Potential complications of biopsy include pneumothorax, sepsis, tumor capsule disruption, spillage and dissemination along the needle track.25,54,55 Bleeding can cause adhesion of the gland to surrounding tissues, making subsequent dissection more difficult, possibly contributing to tumor seeding and local recurrence.55 There is a risk of hemodynamic catastrophe if the lesion is a pheochromocytoma.55,58 These potential adverse events, along with a high proportion of non-diagnostic and false negative specimens,55,58 argues for adrenalectomy without biopsy.55,59 This avoids potential morbidity of two procedures,21,25,50,56,60 especially in the setting of a new adrenal lesion on imaging in an otherwise disease-free patient, particularly within 1 year of primary diagnosis.10,50
Pathophysiology of adrenal metastases
In a landmark autopsy series, the ipsilateral adrenal was affected more often, by larger metastatic lesions, with earlier stage intrathoracic disease, compared to the contralateral.61 This was interpreted as suggestive of a different mechanism of involvement, given that if spread were solely hematogenous, both adrenals should be affected equally.61 Retroperitoneal lymphatic passages may allow direct spread of tumor between the lung and the adrenal,62 and metastatic foci often appear first in the adrenal medulla, which has many lymphatic channels.63 These data suggest that an AM, especially ipsilateral to a lower lobe primary, should be considered regional echelon spread similar to other lymphatic areas.32 By means of the “skip” phenomenon, tumor may miss the first (intrathoracic) nodal station while embedding at the next;61 this has been proposed as the etiology of adrenal OM without apparent mediastinal or hilar disease. Conversely, a contralateral adrenal OM has been proposed as more likely hematogenously deposited via the rich sinusoidal blood supply, potentially portending a worse prognosis.21,24,32,34 Tumor emboli in Batson’s venous plexus can seed either adrenal, depending on transient blood pressure conditions.61 Disease-free interval – that is, whether a metastasis presents synchronously or metachronously – is likely a surrogate for intrinsic biological aggressiveness, and therefore possibly route of spread.33,58 However, some feel that the lymphatic theory of adrenal metastases remains insufficiently supported.50
Open versus laparoscopic approach
In general, the laparoscopic approach has been associated with less intraoperative blood loss, shorter hospital stay, less postoperative opioid use, more rapid return to normal activity, increased patient satisfaction, lower rates of morbidity, and better cosmesis.31,56 However, operatvive (OR) time may be longer, with a steeper practitioner learning curve (suggested at 30–40 procedures).56 Potential complications include: diaphragmatic injury, inferior epigastric injury, pancreatic fistula, pain, hernia, thromboembolism, wound infection, or bleeding; reported incidence is 9–13%.56 At least one case report describes port site and retroperitoneal recurrence.64 Additional adverse events described after laparoscopic adrenalectomy for all indications, not limited to AM, are: 1.2% endocrine complications, 1.2% reoperation rate, 3.6% open conversion, and 0.2% mortality.56 Laparoscopic surgery for an AM may be more likely to require open conversion.65 In comparison, for all indications, the open approach has a higher incidence of: associated organ injury; wound, cardiac, pulmonary and infectious complications; complications overall (35.8%), and mortality (0.9%).56 Extensive discussion of merits and techniques of a laparoscopic versus open approach is beyond the scope of this paper, but this has been excellently reviewed elsewhere.56,66
Symptom palliation
While clinically, most patients are asymptomatic from their AM,26,54 some experience non-specific fatigue, nausea, vomiting, weight loss, and early satiety.67,68 Pain may affect the lower chest, abdomen, back or flank, mimicking hepatic, bone, or chest wall disease.44,67,69 Pain may be severe enough to require opioids and impact negatively on QOL.37 The left adrenal usually extends more cranially than the right; additionally, because the medial border of the left adrenal is closely related to the left celiac ganglion, a left-sided expanding lesion may be more likely to cause pain.68 Adrenal insufficiency is rare, even when AM are bilateral, as over 90% of the gland must be destroyed before there is functional loss.44,68–70 An untreated AM will eventually invade the diaphragmatic crus, celiac axis, kidney, posterior stomach, tail of the pancreas and wall of the aorta, and may cause melena or hematemesis;59 clinically significant hemorrhage into the adrenal is unusual.33,70 Resection or radiation can therefore be palliative27,59,68 (Table 6), or be undertaken to try to prevent future symptomatic progression.10
Interestingly, in Zerrweck’s series of 65 patients (15 NSCLC), on univariate analysis, survival was significantly prolonged if patients were asymptomatic (n=46) versus symptomatic (n=19) from their adrenal metastasis: MS was 52 months versus 17 months, respectively (5-year OS 48% versus 29%, p=0.04). However, this did not retain prognostic significance on multivariate analysis.67
In a series focusing on symptom improvement after RT, 16 (13 NSCLC) patients were treated palliatively for 14 left- and three right-sided lesions (one person had bilateral lesions) between 1972 and 1988.68 Symptoms at baseline included back, flank, epigastric or upper quadrant pain (n=11); and otherwise unexplained nausea, vomiting, or weight loss (n=2). Ten patients received 30 Gy in 10–12 fractions while the remaining had a variety of doses/techniques (range 29.25–45 Gy). Six out of 16 patients had a CR (complete pain relief, no analgesics required), two out of 16 had a good response (marked pain relief, analgesics still required), and four out of 16 had moderate relief (lesser pain relief), for an overall response rate of 75% (12 of 16 patients).68 The remainder had minimal or no pain response. In terms of toxicity, 44% had minimal to moderate nausea and 19% had transient diarrhea. Overall, MS after palliative RT was 3 months: 1 month if other distant metastases were present (seven of 16 patients ), versus 6 months with adrenal-only disease (Table 6).68
Recommendations
Patient selection criteria for aggressive therapy of an adrenal oligometastasis are summarized in Table 7. Surgical resection remains the gold standard, given the large body of data – albeit retrospective – with longer follow up.42 In unresectable or medically inoperable patients, however, SBRT should be considered in experienced centres.35 Survival rates for this highly selected subset of patients compare favorably to both historic controls and to curatively treated unresectable stage III NSCLC patients in the modern era.
Contemporary patients should have 18F-fluorodeoxyglucose (FDG) PET10,27,58 and brain MRI.27 All should have pathologic confirmation of the primary,25 and, in the case of synchronous AM, determination of mediastinal LN status,19,24,28,50 especially nodes with a short axis dimension of 1–1.5 cm.20,26 While surgical or ablative therapy in the setting of N2 intrathoracic nodes remains controversial,33,71 the presence of extra-adrenal OM which can also be approached radically does not appear to be a contraindication.49,67,72 This is due to the absence of a significant difference in MS after aggressive therapy between patients with an isolated AM and those with OM disease, which includes an adrenal lesion.73
Some authors advocate the “test of time” approach in synchronous presentations, utilizing systemic therapy (cytotoxic chemotherapy or targeted agents) for 3–12 months, to allow the natural history of the disease to declare itself.21,26,33,48,59 If, after that period, no evidence of spread is evident on thorough clinical and radiologic reassessment, tumor biology can be considered favorable and aggressive therapy may be entertained.48,59
Eligibility criteria for a laparoscopic approach have been suggested as: tumor <5–6 cm,20,60 <8 cm,30 ≤9 cm (54), or ≤10–12 cm;56,74 absence of caval thrombus;54,74 no involvement of IVC, periaortic LN, renal vein or other adjacent structures;20,30,54,56,60,74 absence of unacceptable cardiopulmonary risk and uncorrectable coagulopathy;56 no morbid obesity;54 and no significant adhesions from prior surgery or trauma.54
Adjuvant systemic therapy should be considered for patients with good functional status to address micrometastatic disease,33,50,71,75 acknowledging that the optimal duration is not defined and the level of supporting evidence is weak. There are insufficient data to support routine postoperative adrenal bed RT at present, although it may be reasonable for R1/R2 resections.
In terms of RT, dose-fractionation schedule is presumed related to treated-metastasis control rates, although again conclusive evidence is lacking.11–12,43 On the basis of the Japanese data demonstrating significantly higher LC after SBRT of NSCLC primary lesions with BED >100 Gy2,76 some recommend this goal in the treatment of AM.11
Conclusions
In patients with an isolated or oligometastatic adrenal lesion secondary to NSCLC, aggressive treatment should be pursued in appropriate patients, if congruent with their wishes. However, it must be acknowledged that it is unclear whether resection or RT in this setting alters the natural history of the disease. Median survival of up to 63 months after surgery and 21 months after SBRT has been reported, with half to one third of patients surviving five years. It is difficult to discern the potential benefit of neo/adjuvant or concurrent systemic therapy and postoperative adrenal bed RT from the data available. Patients being considered for therapy for an isolated AM should be referred in a timely manner, given that early intervention is likely associated with decreased morbidity. 















