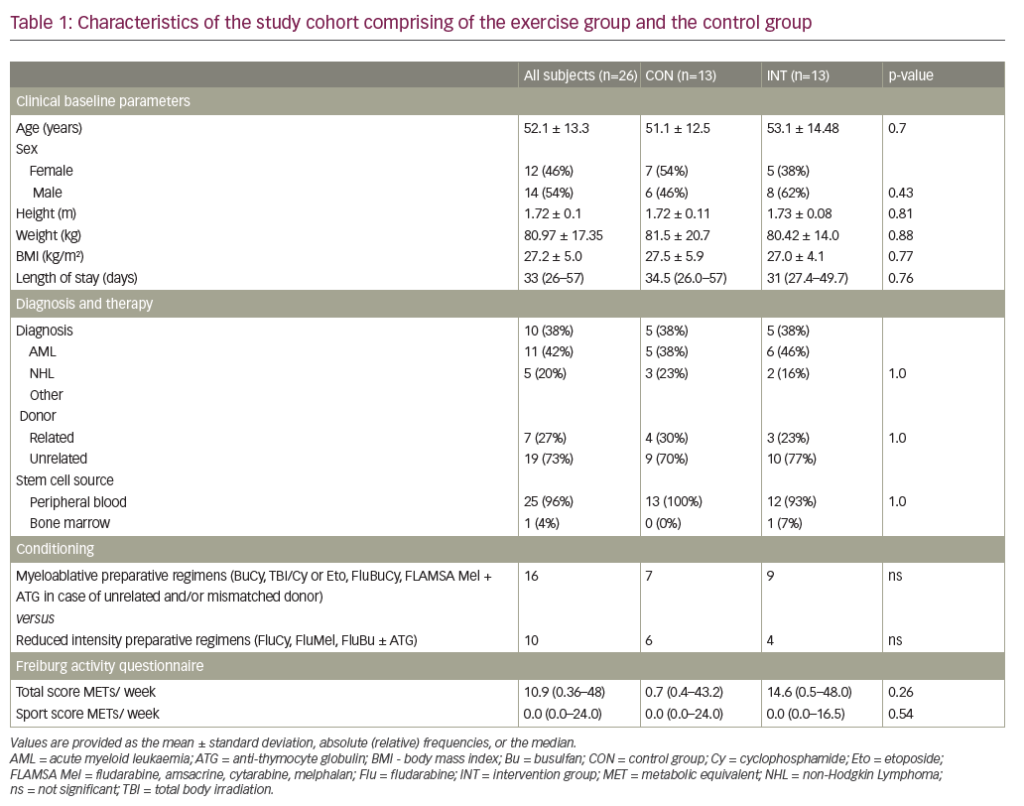
Therapeutic plasma exchange (TPE) is an extracorporeal technique that involves separating a large volume of a patient’s plasma from the cellular components of the blood and replacing it with appropriate fluids.1 In patients with conditions that are induced and/or exacerbated by pathologic factors and toxins circulating in the plasma, TPE can help eliminate symptoms or prevent damage to the organs or systems involved. As such, TPE is often used to modulate the level of circulating antibodies, antigen-antibody complexes, complement components, cytokines, abnormal plasma proteins, cholesterol-containing lipoproteins, plasma-bound toxins and drugs.2–6 Alternatively, TPE can be used to replace a deficient factor in the plasma, such as von Willebrand cleaving factor, or complement factors to treat thrombotic microangiopathies such as thrombotic thrombocytopenic purpura.1 TPE can be used either as a standalone treatment or as an adjunct to conventional therapy.7
TPE can be performed in several ways, either manually or using automated systems. In manual TPE, blood is extracted in repeated cycles and centrifuged to separate the blood cells ex vivo. The supernatant plasma is then discarded and the remainder of the blood returned to the patient with an appropriate replacement fluid.1,4 TPE that is performed using automated devices can be categorised into two distinct types: centrifugal TPE (cTPE) and membrane filtration TPE (mTPE). During cTPE, whole blood is extracted via the same access points and again centrifuged to separate the plasma from cellular components, the supernatant plasma removed and a replacement fluid mixed with the remaining blood and returned to the patient to prevent hypovolemia.1,4 During mTPE, the patient’s blood is pumped through a parallel-plate or hollow-fibre filter. The pores of the filter membranes have a diameter sufficient to allow passage of blood plasma but not the cellular components, thereby enabling plasma removal (Figure 1).4
There are several different types of mTPE devices and cTPE systems currently in use. Differences between cTPE and mTPE systems have been previously reported for key aspects of the procedure, such as procedural times, plasma removal efficiency (PRE), anticoagulation and blood flow rates.1,7–10 However, the majority of these publications present descriptive information; some systems rely on older technologies whose parameters may not be directly comparable with current systems and data from head-to-head comparison studies are more reliable. Spectra Optia® (Terumo BCT, Lakewood, CO, US) is one of the most recent systems to become available and is the only cTPE system to have been evaluated in direct head-to-head comparisons with other mTPE systems. We hereby review current publications that report technological differences in mTPE or cTPE procedures, with a focus on Spectra Optia and data from recent head-to-head comparison studies.
Differences in centrifugal and membrane filtration therapeutic plasma exchange systems
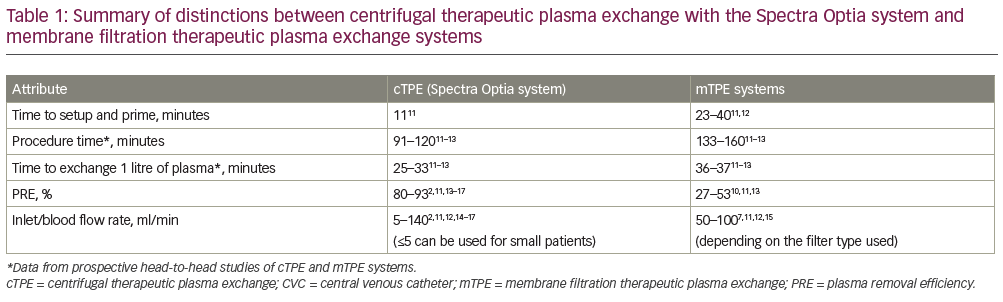
An overview of the technological variances between cTPE and mTPE systems is presented in Table 1.11–7 One of the clearest differences between the cTPE and mTPE systems is the amount of time required to perform the TPE procedure. This includes both the time to set up and prime the system and the time to carry out the procedure itself. Individual differences are discussed in more detail in the following sections.
Setup and priming time
Few studies have reported information related to the time taken to setup and prime the equipment prior to TPE procedures. In general, cTPE systems take less time to setup and prime as they do not work with a balancing system, unlike many mTPE systems, and the calibration of these balances limits procedural speed. Of the information available, a head-to-head study comparing the Spectra Optia and mTPE Diapact® CRRT system (B Braun Avitum AG, Melsungen, Germany) reported a shorter time to set up and prime the Spectra Optia system (n=27; 11 ± 1 minutes versus 23 ± 2 minutes, respectively).11 A longer time of ~40 minutes has also been reported for setting up and priming the mTPE Prisma® system (Baxter International, Deerfield, IL, US) using a TPE 2,000 set.12
Plasma removal efficiency
PRE is an established metric used to analyse the performance of an apheresis device during a TPE procedure. It represents the fraction of plasma that has been removed in a TPE procedure in relation to the plasma that has been processed. PRE values should theoretically remain independent of the volume of blood processed. However, due to the time taken for blood to replace the priming fluid in the device at the start of the procedure (during which time blood is being processed but no plasma is removed), short TPE procedures may tend to have lower PRE values than long procedures.
Variations in plasma removal efficiency between centrifugal and membrane filtration therapeutic plasma exchange systems
Head-to-head comparisons of the cTPE Spectra Optia system and two mTPE systems (Diapact [n=27], OctoNova®; Diamed, Cologne, Germany; and Plasmaflo OP 05W filter [n=20])11,13 showed significantly higher PRE values with Spectra Optia (83% versus 53%; p<0.0001,11 and 84% versus 27%; p<0.05,13 respectively). This trend is consistent with previous publications (Table 1), as are the PRE values for Spectra Optia, with several individual studies confirming PRE values ranging from 80.0–92.5%.2,14–17 The impact of this large difference in PRE between cTPE and mTPE is substantial, as it means the procedure time with mTPE has to be considerably longer than cTPE in order to remove the same volume of plasma (see next section for further details).1 Blood inlet flow rate is often increased in mTPE to reduce the procedure time to an acceptable level and to provide adequate pressure across the filter membrane to ensure successful separation (e.g. >40 ml/min, depending on the filter type).7,11,12,15 As a result, although both mTPE and cTPE procedures can be performed using peripheral veins, a central venous catheter, arteriovenous fistula/grafts or implanted ports, mTPE devices often require a central venous catheter to obtain adequate blood flow rates.7,9 This might confer additional safety risks, as TPE using peripheral venous access has been shown to reduce the risk of infection (bacteraemia) compared with a central venous catheter.9,10 Additionally, it might limit the choice for optimal venous access due to specific patient and procedural requirements, while for cTPE systems the flexibility is greater. The higher PRE associated with cTPE compared with mTPE can also limit the choice of anticoagulant; with cTPE most of the anticoagulant would be isolated from the blood and discarded, whereas with mTPE more of the anticoagulant would be returned to the patient. If citrate is used in an mTPE system, this could lead to an increased risk of citrate toxicity (resulting in hypocalcaemia and electrolyte abnormalities),8 thus anticoagulation in mTPE systems is usually limited to heparin.7,10
Procedure time
The cTPE procedure time can be greatly affected by blood flow rate, as well as other factors such as the haematocrit, total blood volume of the patient, anticoagulation ratio and infusion rate. This has resulted in a wide variation in procedure times reported in cTPE studies (81–128 minutes) and makes between-study comparisons with mTPE challenging.14–20 However, three head-to-head studies have reported data on the time taken to perform Spectra Optia and mTPE procedures.11–13 Flow rates still varied between studies (Spectra Optia: 54–81 ml/min; mTPE systems: 82–150 ml/min), but despite this the Spectra Optia procedure times were consistently shorter than mTPE procedure times (91–120 minutes versus 130–160 minutes; Spectra Optia versus Diapact, Prisma and OctoNova; n=27, n=3 and n=20, respectively).11–13 In the latter example (OctoNova), flow rates with the cTPE system were kept within the low range that is normally used with peripheral vein access.13 Interestingly, even with these lower flow rates, a shorter procedure time was still reported with the cTPE system.13 These findings are also supported by real-world data from a retrospective chart review study of 912 TPE procedures performed in 185 patients at a tertiary care hospital (Hannover Medical School, Germany) between 1 January and 31 December 2012.21 Overall, a significantly shorter procedure time was reported with cTPE compared with mTPE (120 minutes versus 135 minutes, respectively; p=0.007).21 As mentioned above, the lower PRE with mTPE systems is believed to be compensated by higher flow rates (but with the lower flexibility of peripheral vein access), though this appears to be only a partial compensation.
Standardised data on the time taken to remove 1 litre of plasma were also calculated and are presented in Table 1. This standardisation was performed to adjust for variations in the total plasma volume of the patient and provide a more robust comparison than procedural time alone. Overall, 1 litre of plasma was removed in 25–33 minutes with the Spectra Optia system and 36–37 minutes with the mTPE systems.11–13
In conclusion, it is estimated that the whole TPE process (setup, priming and procedure) with the Spectra Optia system may take as little as two-thirds of the time required by some mTPE systems.11 As well as making the procedure more amenable to the patient, shorter procedure times also aid in achieving the recommended TPE dose (1.0–1.5 times the individually calculated total plasma volume of the patient)13,22 within the allotted clinic visit.
Safety
Adverse events
Previous studies have reported adverse events with either cTPE or mTPE procedures, though direct comparisons between the systems were not drawn and much of the data involved older technologies.23–26 Overall, adverse events during any type of TPE procedure were shown to be rare.23–26 More recently, a report from the World Apheresis Association registry has provided a comprehensive assessment of adverse events associated with TPE procedures.27 In total, 50,846 procedures in 7,142 patients were assessed, adverse events graded by severity and comparisons drawn between various procedural aspects, including the use of cTPE or mTPE. Overall, adverse events were infrequent: mild events occurred in 2.4% of procedures, primarily due to access (54%) or hypotension (15%); moderate events occurred in 3.0% of procedures, which included primarily tingling (58%), urticaria (15%) and hypotension (10%); and severe events occurred in 0.4% of procedures, primarily hypotension/syncope (32%) and urticaria (17%).27 With regard to the type of procedure, mTPE was associated with significantly more adverse events than cTPE both overall (11% versus 6%; p<0.0001; odds ratio [95% confidence interval]: 1.8 [1.5–2.3]) and when stratified by event severity (mild: 2.9% versus 1.6%; p<0.001, moderate: 6.6% versus 3.8%, p<0.001 and severe: 1.0% versus 0.7%; p<0.05).27 In a recent head-to-head study in an intensive care unit setting, similar rates of adverse reactions were reported for both cTPE and mTPE procedures (23.9% versus 31.7%; p=0.19),28 though the high adverse event rates suggest this may be due to the intensive care setting.
Specific adverse events have also been identified that only occur with some mTPE systems. These include adverse events related to the use of a filter, especially with high blood flow rates (and therefore high transmembrane pressures), such as haemolysis, the rupturing of filter fibres leading to contamination of plasma, and the activation of complement and white blood cells on the artificial membrane itself.4,7,29,30 However, there is evidence that complement activation may be due to the specific type of filter and might therefore be avoided if an appropriate mTPE filter is used.29
Anticoagulation and clotting events
In general, cTPE devices use citrate (e.g. acid-citrose dextrate formula A) as an anticoagulant, whereas mTPE systems tend to use heparin.7,10 Both methods of anticoagulation have their inherent risks, with the potential for an increased risk of bleeding and thrombocytopaenia with heparin31,32 and an increased risk of hypocalcaemia with citrate.8 Heparin-induced adverse events are rare but serious, with heparin-induced thrombocytopaenia occurring in 0.5–5.0% of procedures.31 Citrate-induced adverse events are also rare (≤1.2% of procedures),8 but tend to be mild and self limiting; as with dialysis, citrate-only anticoagulates the extracorporeal circuit and is neutralised as soon as it is mixed with systemic blood, avoiding any increased risk of systemic bleeding.33 In addition, citrate-induced adverse events may be effectively treated with prophylactic calcium.9,34 Reflecting this, in three studies involving a total of 50 patients receiving both cTPE and mTPE, only one case of possible mild symptomatic hypocalcaemia was reported with the Spectra Optia system.11–13 Citrate reactions and hypocalcaemia have also been reported for mTPE systems as well as cTPE systems,28 as citrate is often present in apparent concentrations in the replacement fluids used (± 4.4 mmol/l in albumin and 17 mmol/l in fresh frozen plasma).35
Clotting and clumping around filters poses an issue with mTPE systems that clearly differs from cTPE systems. In a head-to-head case report of the Spectra Optia and Prisma systems (n=3), nine mTPE procedures required 13 filters due to clotting events, while no clotting complications were reported with Spectra Optia and only one disposable equipment set was required for each procedure.12 While the high clotting rate in this study may be attributable to the varying heparin doses used, other studies have also reported that clotting resulted in premature termination of the procedure or the use of an additional filter set in up to 23% of mTPE procedures.3,11,13,28,36,37 Similar events have not been reported for Spectra Optia TPE procedures.11–13 While clotting and clumping could potentially happen with either system, these findings are not surprising due to the differences in their design. More specifically, when a clotting event does occur with an mTPE system, usually due to the limited diameter of the filter fibres, this appears to result in a premature end to the procedure or the need for a new filter or disposable set more frequently than with cTPE systems.11–13 Further, each time a clotting event occurs where the filter is lost, the blood components in the filters cannot be returned to the patient, which could lead to additional safety considerations with mTPE systems. As mentioned previously, cTPE clotting events in peer-reviewed literature have not been reported so far, but it is possible that they might occur in patients with specific indications that could increase the chance of clotting or precipitates, such as cryoglobulinaemia (especially at temperatures below 37°C).38 Specific measures should be taken to avoid clotting in these cases.
Platelet loss
Platelet loss is another patient safety concern during TPE, as platelets have the lowest specific gravity of all human blood cells and therefore the highest probability of being removed with plasma during the procedure. In general, platelet loss can be calculated using the formula:

It has been previously reported that cTPE devices confer a greater risk of platelet loss than mTPE devices.4,39 However, reported cTPE values range from 3–16% for the COBE® Spectra system (Terumo BCT, Lakewood, CO, US)15,16,19,40 and 8% for the MCS®+ system (Haemonetics®, Braintree, MA, US).2 Spectra Optia also reveals a lower-to-similar level of platelet loss (1.0–10.5%).11,13,15,16,19,40 These findings were supported by results of two recent head-to-head studies comparing cTPE and mTPE systems. In one study, similar levels of platelet loss were reported for the Spectra Optia and Diapact systems (10.5 ± 16.5% versus 9.7 ± 15.5%, respectively; p=non-significant; n=27),11 whereas in another study, platelet loss with Spectra Optia was almost half that observed with the OctoNova mTPE system (7 ± 9% versus 15 ± 9%, respectively; p<0.05; n=20).13 The slightly higher than expected platelet loss reported by Kes et al. (10.5%) may be due to the use of a greater mean blood flow rate (80 ml/min) compared with earlier studies and the head-to-head study by Hafer et al. (54 ml/min).2,11–13,14,16,17 Nevertheless, platelet loss overall remains acceptably low (~10%) with both cTPE and mTPE systems.
Disease management
It has been previously reported that mTPE systems are less effective than cTPE systems for the removal of higher molecular weight proteins such as immunoglobulin and immune complexes.4 However, in two recent head-to-head studies both cTPE and mTPE systems (Spectra Optia, Diapact, OctoNova) showed similar effectiveness in removing immunoglobulin G and/or M,11,13 with one study reporting an immunoglobulin G removal efficiency of 72 ± 10% with Spectra Optia and 69 ± 9% with Diapact.11,13 On the other hand, the latter publication also reported that fibrinogen was more efficiently removed with the cTPE than with the mTPE system (Diapact: 62.95 ± 16.14%; Spectra Optia: 72.32 ± 8.54%). While other immune complexes and plasma bound proteins have not been analysed in head-to-head comparisons, depending on the filters used, resulting sieving coefficient differences between cTPE and mTPE systems might exist.
It should be noted that cTPE systems are unable to perform continuous renal replacement therapy (CRRT) procedures, unlike mTPE systems. This is because cTPE systems are specifically designed to perform apheresis procedures, whereas mTPE can be performed using either CRRT or a haemodialysis machine.5 On the other hand, because of their specialisation, cTPE systems are able to perform most types of (therapeutic) apheresis procedures (e.g. peripheral blood stem cell collections, white blood cell/platelet depletion), something which is not possible with mTPE systems.5
Conclusions
This review summarises comparative information between mTPE and cTPE systems. While technical and performance parameters for various TPE systems have been reported previously, the recent publication of several head-to-head studies directly comparing cTPE and mTPE systems has increased the understanding of their technical and procedural performance.11–13 The majority of technical variables favour the Spectra Optia cTPE system over mTPE systems, with a low risk of clotting events and clear benefits seen in PRE and procedure time. In mTPE systems, the lower PRE is often somewhat compensated (though not entirely) by using higher flow rates, which has as an extra disadvantage that the choice for venous access type, more specifically peripheral access, becomes limited.
Both cTPE and mTPE procedures are generally safe and well tolerated, though lower rates of adverse events were reported with cTPE systems in a comprehensive analysis of data from the World Apheresis Association registry.27 Platelet loss with the Spectra Optia system was shown to be similar or reduced compared with mTPE systems. mTPE devices are also, in general, more associated with the use of heparin while cTPE systems are mostly performed by citrate as an anticoagulant. However, citrate is often abundantly present in the replacement fluid during procedures of both device types. As a result, hypocalcaemia has been observed as an adverse event during both cTPE and mTPE procedures.
There is currently insufficient evidence in published literature from which to draw robust conclusions on the efficiency of the removal of disease mediators or other molecules with either type of system. Indeed, although some publications mention that there are differences, especially for higher molecular weight proteins, the data are limited and further research is required.
Regional differences in the use of either type of system indicate that other factors, such as historical use and reimbursement costs, may influence TPE treatment decisions.41 In addition, the need to perform other procedure types in the facility where the TPE system is required may also influence the type of TPE system selected. By reducing the occurrence of clotting events, it is reasonable to assume that cTPE would have reduced disposable costs compared with mTPE systems. Similarly, a reduced procedure time and lower adverse event rates would also be expected to reduce the cost of treatment over the long term. In conclusion, there are several reasons to suggest the use of cTPE or mTPE systems based on the differences described here.