Severe haemophilia A (HA), defined as baseline factor VIII (FVIII) levels of <1%, results in traumatic and spontaneous bleeding episodes, which occur primarily in the joints, in addition to the muscles, soft tissue and the central nervous system. Prophylactic treatment is needed to prevent complications such as joint arthropathy.1 Treatment for HA has historically been achieved through intravenous FVIII replacement therapies, which can be burdensome, costly and immunogenic.2 The development of antibodies to FVIII products occurs in 25–40% of patients and leads to decreased function, and complicated treatment strategies are needed to eradicate inhibitors.2,3 In recent years, novel therapeutic approaches have been investigated to achieve similar or better haemostatic control than previous therapies with reduced frequency of infusions. Strategies such as modified FVIII molecules, substitution molecules and the targeting of natural anticoagulation proteins for haemostatic balance are under investigation, some of which have shown great efficacy.2,4
Efanesoctocog alfa is a novel, von Willebrand Factor (VWF)-independent recombinant FVIII therapy approved by the US Food and Drug Agency in 2023 for prophylaxis and on-demand treatment of bleeding episodes in patients with HA.5 Efanesoctocog alfa is the first FVIII product not subject to the half-life limitations of VWF coupling, which had previously limited the half-life of FVIII therapies.6 Efanesoctocog alfa is independent of VWF binding due to the addition of the VWF binding site to the FVIII molecule, in addition to two XTEN stabilizing proteins.7 The pharmacokinetics and safety of efanesoctocog alfa were initially investigated in phase I and I/IIa studies for adults with severe HA and demonstrated superior half-life to currently approved standard and extended half-life products.8,9 The XTEND-1 trial (ClinicalTrials.gov identifier: NCT04161495), a phase III study, evaluated the efficacy of efanesoctocog alfa in previously treated patients with severe HA aged 12 years or older.7 The XTEND-1 trial results were pivotal, showing that once-weekly prophylaxis with efanesoctocog alfa dosed at 50 IU/kg body weight improved bleed prevention compared with prestudy FVIII therapies and increased FVIII activity levels to the normal to near-normal range for the majority of the week.7 Efanesoctocog alfa must be administered intravenously, thus requiring the patient or the caregiver to be comfortable with in-home infusions or have the ability to access a medical centre for infusions. One-stage assays are recommended to monitor FVIII activity levels in patients receiving efanesoctocog alfa.10
The 2023 International Society on Thrombosis and Haemostasis Congress late-breaking session “Efanesoctocog alfa prophylaxis for previously treated patients <12 years of age with severe hemophilia A” by Lynn Malec presented results from the XTEND-Kids study (ClinicalTrials.gov identifier: NCT04759131).11 XTEND-Kids was an open-label, multicentre, phase III trial evaluating outcomes of efanesoctocog alfa use in previously treated children with severe HA younger than 12 years of age.11 The study enrolled 74 participants, who received weekly prophylaxis using efanesoctocog alfa at a fixed dose of 50 IU/kg intravenously. The primary endpoint was FVIII inhibitor development, defined as the presence of neutralizing antibodies ≥0.6 BU/mL, and was confirmed by a second sample drawn 2–4 weeks later, with a reported incidence of 0.0% (95% confidence interval 0.0–4.9%).
Secondary endpoints of the XTEND-Kids trial included annual bleed rate (ABR), pharmacokinetics data, treatment of bleeds, safety and tolerability, and perioperative management. Efanesoctocog alfa prophylaxis demonstrated effective prevention of bleeds, with an overall ABR of 0.89; when subdivided by age, ABR was 0.48 in participants <6 years of age and 1.33 in those aged between 6 and under 12 years. When analysing treated bleeds, 64% of XTEND-Kids participants had zero bleeding episodes, 82% had zero joint bleeds and 88% had zero spontaneous bleeds. Overall and annual joint bleed rates were found to be lower in a sensitivity analysis set. The ABR in participants aged 6–11 years was higher than in those aged <6 years despite a longer half-life. This difference may be attributable to increased activity levels in the older age group.
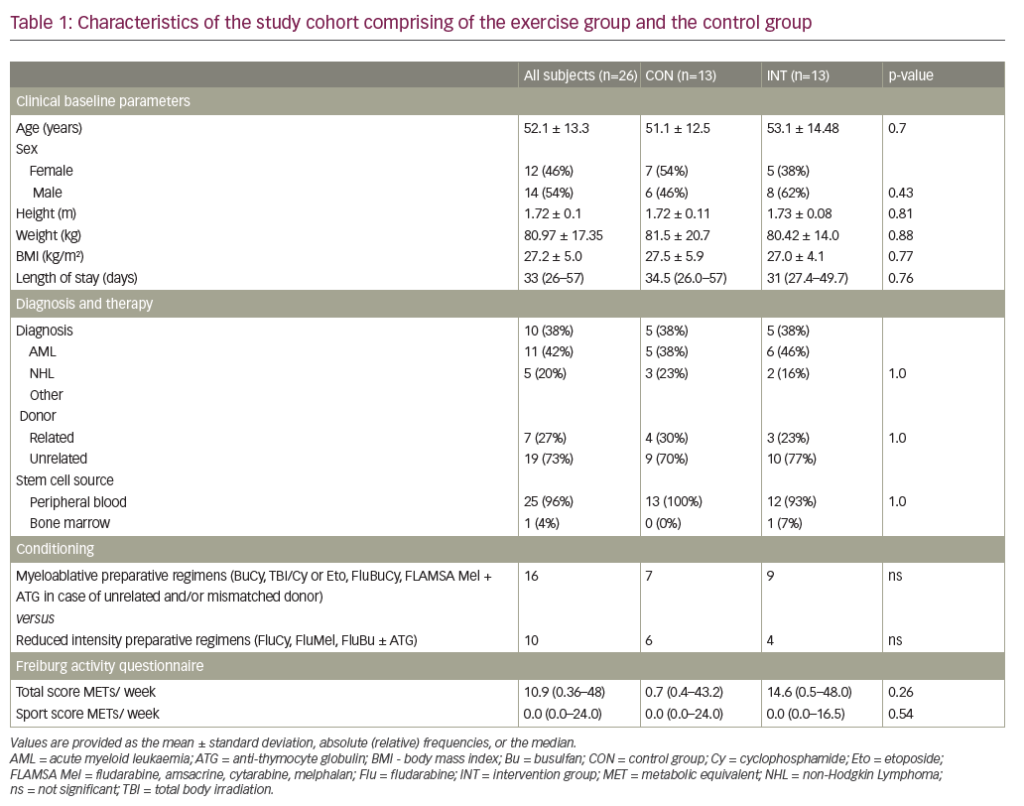
In addition to prophylaxis, efanesoctocog alfa was also shown to be efficacious in the treatment of bleeds. During the XTEND-Kids study period, there were 43 treated bleeds, 95% (41) of which resolved with just one 50 IU/kg infusion of efanesoctocog alfa. Two participants underwent major surgical procedures during the study period and had excellent haemostatic responses with preoperative dosing of 50 IU/kg. Pharmacokinetic analysis of participants <6 years of age (n=19) showed a half-life of 38.0 h after the first dose compared with 42.4 h in participants aged 6–11 years (n=18). Pharmacokinetic profiling confirmed that efanesoctocog alfa allowed for weekly prophylactic dosing in the older age group, as steady-state trough levels were >10 IU/dL at 7 days (Table 1). Although the XTEND-Kids data showed slightly lower FVIII levels than those seen in the XTEND-1 study, this was expected given the known pharmacokinetic differences between younger children versus adolescents and adults.12 The mean number of exposure days for patients in the XTEND-Kids trial was 52.5, and no reports of serious allergic reactions or thrombotic events were reported.
Table 1: Mean factor VIII activity after the first dose in the pharmacokinetics subgroups of the XTEND-Kids trial as per activated partial thromboplastin time one-stage clotting assay
|
Subgroup |
Day 0 |
Day 3 |
Day 7 |
|
<6 years (n=19) |
140% |
34% |
6% |
|
6–11 years (n=18) |
111% |
41% |
7% |
These late-breaking data from the XTEND-Kids trial provide important information on the use of this novel FVIII product in participants younger than 12 years of age. As the US Food and Drug Administration approved efanesoctocog alfa for individuals of all ages, these data are essential for supporting its use among younger patients and helping increase provider confidence. Further data on previously untreated patients, who are at the highest risk of inhibitor development, are needed. The encouraging results observed in the XTEND-1 and XTEND-Kids trials provide clinicians with additional information to counsel families on the emerging landscape of FVIII therapies and help guide patient-centred decisions around prophylaxis.
Participants in the XTEND-Kids study were able to achieve near-normal FVIII levels with less frequent administration than prior FVIII products, substantially decreasing the burden of treatment while still providing high levels of protection, making efanesoctocog an attractive prophylaxis option for paediatric patients. Emicizumab, a non-FVIII subcutaneous option for the treatment of HA, has become increasingly popular for paediatric prophylaxis, yet it still requires FVIII replacement for the treatment of bleeds and in perioperative settings.13 There may be a proportion of patients who switch from emicizumab to efanesoctocog due to different product profiles with regard to route of administration and level of haemostatic coverage. Patients who remain on emicizumab, as well as patients with mild disease not on prophylaxis, may also benefit from the excellent bleed control and perioperative haemostasis reported in the XTEND-Kids trial.