Renal cell carcinoma (RCC) represents 2–3 % of all cancers, and is responsible for 13,570 annual US deaths.1 Surgical resection of localized RCC can be curative, but disease recurrence eventually occurs in many patients, at rates that can be directly related to features including tumor size or grade. In addition, many patients are diagnosed with either locally advanced and unresectable or metastatic disease at the time of initial presentation. Subtypes of tumors arising in the kidney are classified according to the World Health Organization (WHO) classification system.2 The most common subtype is clear cell RCC, which accounts for over 80 % of malignant, nonurothelial kidney tumors.3 The molecular profile of this clear cell RCC is heterogeneous.4 Recent studies suggest the presence of two major molecular subtypes, which may explain the variable clinical course and response to therapy among patients with clear cell RCC.5 However, even within a single patient, substantial differences of gene expression have been observed within the primary tumor, or between the metastases and the primary.6,7 Heterogeneity of patient clinical features is acknowledged to be a dominant factor of the incident RCC population, with impacts on overall survival (OS) from the disease features greater than many treatment choices. Ultimately, however, around 70 % of RCC patients develop metastases, and until the introduction of targeted therapies, the 5-year OS of metastatic RCC (mRCC) was around 5–10 %.8
Over the last decade, an increased understanding of tumor biology helped drive development of targeted therapies for mRCC.9 These easily administered oral and intravenous therapies, targeted against intracellular signaling pathways such as those activated by vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin (mTOR) pathway, have revolutionized the way mRCC is treated. However, despite the advent of these newer agents, and a significant improvement of the median OS from 13 months in 200210 to about 28 months in 2012 to 2013,11,12 almost all patients eventually experience disease progression, and die of their disease. The management of mRCC therefore remains a therapeutic challenge.
Durability versus Chronic Treatment
Long before the clinical use of targeted therapies, immunotherapy was extensively studied as a therapeutic approach to mRCC. In nonrandomized clinical trials, high-dose interleukin-2 (HD IL-2) demonstrated durable complete responses, achieving recurrence-free, treatment-free survival exceeding 10 years in around 8 % of patients with advanced or mRCC who were treated. Despite the established benefit of HD IL-2, and that patients progressing through IL-2 generally remain eligible for subsequent targeted therapies, the population treated with immune therapy with IL-2 remains limited. The bases for the limited utilization of HD IL-2 include toxicity, cost, hospital time, and the lack of benefit in the majority of patients. Regarding toxicity, short-term side effects with IL-2 can be contrasted with more chronic but less severe side effects associated with open-ended treatments on targeted therapies. Regarding drug costs, the overall cumulative cost with targeted therapy can be similar or more than treatment with HD IL-2, particularly for the patient with disease features that predict for multiyear survival. Of course, the duration of treatment used, the patient selection, and the fact that IL-2 is followed by targeted therapy in most cases makes comparisons difficult.
Almost all mRCC patients on targeted therapies eventually experience disease progression, with long-term remissions represented only in isolated cases, and not in the larger phase III studies. Increased understanding of the limitations of the targeted therapies over the last few years and recent advances in the cancer immunotherapies in general has led to a resurgence of interest in the investigation of immunotherapy as a major treatment strategy, with HD IL-2 and other approaches such as checkpoint inhibition and vaccination being investigated in the treatment of mRCC.13,14 This article will review current research investigating immunologic therapy in mRCC with emphasis on the approved therapy with IL-2.
Immunotherapy as a Therapeutic Approach to Metastatic Renal Cell Carcinoma
For the most part, RCC has not shown a significant response to traditional cytotoxic therapy, and this helped to drive interest in other approaches; immunotherapy is known to result in rare but dramatic responses in some RCC. The observation of mRCC apparently showing spontaneous regression in placebo groups of clinical trials is presumably a result of the isolated events of the host immune response waxing stronger over time.15
The antigenic features of certain cancers that make them responsive to immunotherapy are poorly understood and are the subject of considerable research. Melanoma is a particularly immunogenic cancer, for which many tumor antigens are well characterized. An enhanced understanding of the differences in antigenic features between mRCC and melanoma would accelerate the development of immunotherapies for mRCC. One potential difference lies in tumor-associated antigens (TAAs) that trigger the cell-mediated immune response. Tumor-infiltrating lymphocytes (TILs) are found in high numbers in RCC tumors; however, they are not directed at TAAs and have not demonstrated clinical efficacy in mRCC, a contrast with the melanoma experiences. Treatment with CD8+ TILs did not improve response rate or survival in RCC patients treated with lowdose IL-2 after nephrectomy.16 Reasons for lower frequency of clinically useful immune response of the treatment may include a lack of TAA or of more tumor-induced local or systemic immunosuppression. Although preclinical evaluations suggested that adoptive cell transfer (ACT) could be a promising approach in mRCC patients,17 a recent meta-analysis identified five hindrances to the lack of success of such approaches, including high degree of personalization, unsuitable response assessment criteria, inadequate identification of TAAs, lack of effective combination treatments, and insufficient attention paid to the quality of ACT products.18
Removal of the primary tumor by surgical resection may cause a change to the immunologic environment. Since the primary tumor bulk is immunosuppressive, removal of the tumor has a theoretically favorable immunotherapeutic effect.19 Clinical studies have demonstrated that early nephrectomy in patients with good performance status confers a survival advantage.20,21 However, it is important to consider individual risk assessments in any decisions around nephrectomy.22–24 There are also opportunities in the context of clinical trials to integrate nephrectomy with subsequent immunotherapy, including IL-2, but also with others such as dendritic cell (DC)-based vaccines based on primary tumor tissue.25
Cytokine Therapy
Interferon Alpha
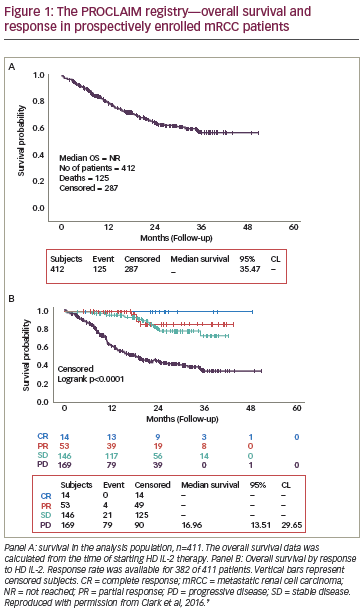
The greatest body of clinical experience with immunotherapy in mRCC is in the use of cytokine therapy with interferon alpha (IFN-a) or HD IL-2 (Proleukin®), which are the only immunotherapies recommended in treatment guidelines (see Figure 1)26 and have been a standard of care for over 20 years. However, IFN-a has only a modest impact on survival in selected patients (nonbulky pulmonary and/or soft tissue metastases with performance status ratings of 0–1, according to the Eastern Cooperative Oncology Group [ECOG] rating scale, and no weight loss). In clinical trials, IFN-a proved inferior to HD IL-2,27 to sunitinib,28 and to the combination of bevacizumab + interferon.29,30
Interleukin-2
IL-2 (aldesleukin) is a recombinant protein that has numerous antitumor actions including enhancing cytotoxic immune cell functions; limiting tumor escape mechanisms such as defective tumor cell expression of class I or II molecules or expansion of regulatory T cells (Tregs); and indirect effects on the tumor microenvironment.31 IL-2 received US Food and Drug Administration (FDA) approval in 1992 for the treatment of mRCC, based on the results of seven phase II clinical trials.32 A subsequent randomized clinical trial (n=156) demonstrated that outcomes could be improved with the higher dosage (HD IL-2).33 A phase III clinical trial (n=192) found superior survival with intravenous HD IL-2 compared with subcutaneous IL-2 plus IFN-a (IL-2/IFN-a). The response rate was 23.2 % for HD IL-2 versus 9.9 % for IL-2/IFN-a (p=0.018). The median response durations were 24 and 15 months, respectively. The median survivals were 17.5 and 13 months (p=0.24).27 A 20-year analysis of patients treated at the National Cancer Institute from 1986 to 2006 (n=259) showed an overall response rate of 20 % with complete response in 9 % of patients with mRCC after treatment with HD IL-2. Median survivals of the partial responders and nonresponders were 39.1 and 15.1 months, respectively. The median survival of the complete responders had not yet been reached after several years, at the time of last follow-up.34
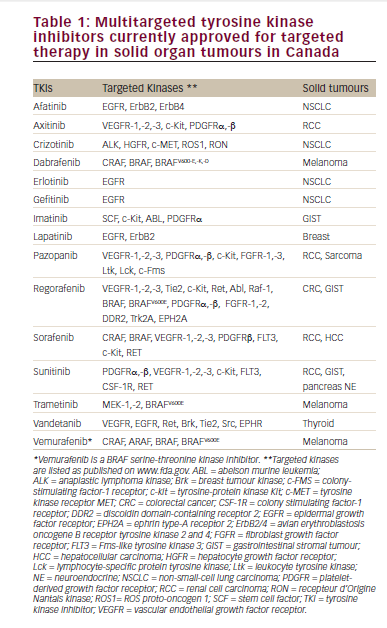
In addition to those patients achieving complete response, HD IL-2 may confer a clinical benefit to an additional ~40 % of patients who achieve partial response or stable disease and experience clinically meaningful survival rates with median OS of 38 to 42 months among the patients who receive it.35–37 Response rates to treatment with VEGF pathway drugs, in patients with prior IL-2 therapy or prior interferon therapy, appear about the same in nonrandomized, cross-trial comparison to those seen in patients with no prior therapy (see Table 1). A direct prospective comparison has not been conducted to address that matter specifically.
The use of HD IL-2 is associated with adverse effects (AEs), mainly because of capillary leak syndrome.44–46 Patients with mRCC should therefore be carefully assessed for suitability in terms of cardiovascular and the pulmonary status to undergo treatment with HD IL-2.47
Optimizing Response to High-dose Interleukin-2
Since HD IL-2 is a therapy capable of producing durable complete responses in mRCC, a key goal has been to establish a prospective way to define the subset of patients with the best chance of response, or conversely, to exclude those unlikely to respond. A number of studies have attempted to identify clinical and tumor factors that may affect responsiveness of certain patients to HD IL-2. Response rates to HD IL-2 are highest in clear cell carcinomas with more than 50 % alveolar features and no granular or papillary features.48 In a case series where patients were stratified according to histology, the overall response rate was almost doubled in the subgroup of patients with favorable histologic features (less than 10 % papillary features and at least one of: >50 % alveolar/solid or >50 % clear cell features).47 In an extension of this study (n=103), the overall response rate was 57 % with complete response in 22 %.49
Basal levels of the immunosuppressive cytokines may also be important: higher C-reactive protein level has been associated with poor survival in mRCC patients treated with HD IL-2.50
The HD (IL-2) ‘Select’ trial (n=120) was designed to determine if the response in mRCC with favorable predictive features was significantly higher than a historical, unselected population.51,52 The investigator-assessed response rate was 29 % (5.8 % complete responses; 23.3 % partial responses) and was significantly greater than the historical response rate (p=0.0009). The median progression-free survival (PFS) was 4.4 months and 20 responses were ongoing at the time of analysis (range 4 to 35+ months). The study also identified a group of patients with clear cell RCC and performance status of 0 that showed a doubling of the historical response rate 30 % (p=0.0004). However, clinical and pathologic features (e.g. Survival after Nephrectomy and Immunotherapy [SANI] score and histology) did not identify patients likely to benefit from HD IL-2, but may identify patients unlikely to benefit from HD IL-2. The SANI score is composed of lymph node status, constitutional symptoms, location of metastases (site other than lung or bone or multiple sites of metastases), sarcomatoid histology, and thyroid-stimulating hormone (TSH) level.53 Despite an observation in a nonrandomized series that high tumor expression of carbonic anhydrase 9 (CAIX) may be predictive for benefit from HD IL-2,54 staining for CAIX did not define a subset with better overall response frequency. The expression of programmed death ligand-1 (PDL-1) on tumor did identify a higher response group.51 Conversely, an analysis of the COMParing the efficacy, sAfety and toleRability of paZopanib vs. sunitinib (COMPARZ) phase III trial comparing pazopanib and sunitinib in mRCC found that increased expression of PDL-1 was associated with shorter OS.55
Further studies to investigate tumor and host-derived predictive biomarkers are ongoing. A recent study (n=85) found the following predictors of increased OS with HD IL-2: fewer and less-severe AEs; higher baseline weight; male gender; and the ability to tolerate a higher cumulative dose of IL-2. Of note, increased age did not predict poor outcome, and therefore should not be considered in itself as a contraindication to treatment with HD IL-2 in otherwise eligible patients with good performance status.56 Further prospective studies are required to confirm these findings.
Tumors express tumor-associated antigens that are captured by antigenpresenting cells (APCs), which prime naïve T cells to become antigen-specific cytotoxic T lymphocytes (CTLs). Immunotherapies therefore aim to induce the production of CTLs. In addition to CTLs, Tregs are increased in individuals with cancer, and are associated with suppression of immune response and cytotoxicity.57,58 However, the paradigm that Tregs are disadvantageous for the control of malignancies is now under scrutiny.58,59 A recent study of melanoma patients found that patients with higher levels of Tregs following treatment with IL-2 had better clinical outcomes.60 Further research is required to fully elucidate the significance of the levels of Tregs in patients with mRCC.
Other studies have investigated modifications of the dosage schedule in order to maintain efficacy while minimizing AEs. In a retrospective chart review of 41 consecutive metastatic melanoma (n=33) and renal cancer (n=8) patients, a modified twice-daily dosing schedule that limited the total number of doses per course to eight showed durable clinical activity in melanoma but not mRCC.61 However, a review found that IL-2 is capable of stimulating different populations of T cells in humans to induce either T effector or Treg responses and hypothesized that alterations in administration schedule of HD IL-2, may have a positive impact on cytotoxicity.62 Another single-arm study (n=36) investigated a novel dosing schedule for HD IL-2, monitoring the effect of intermittent pulses of IL-2 on the expansion of activated cell populations in the immune response. The target dose was achieved with an AE profile similar to that expected for conventional schedules, but with an apparently reduced requirement for vasopressor support. In addition, changes were observed in the ratio of DCs to myeloid-derived suppressor cells (MDSC), specifically increases in MDSCs. Patients who had more favorable outcomes had high pretreatment DC:MDSC ratios. This may also be used to predict response to HD IL-2.63 These studies merit further investigation.
Other cytokines have had limited success in mRCC.64 However, new agents are being developed for mRCC. The combination of IL-21 plus sorafenib demonstrated antitumor activity in a recent phase I/II clinical study.65 IL-15 is also in clinical development.66
New and Emerging Combinatorial Immunotherapeutic Approaches
A summary of agents in clinical development is given in Table 2.
Combined and Sequential Regimes with High-dose Interleukin-2
Numerous clinical studies are investigating the use of HD IL-2 in combination therapy with bevacizumab67 and with sorafenib68 in sequential regimens.69 The combination of adjuvant 5-fluorouracil, IFN-a, and IL-2 in the adjuvant setting was associated with significant toxicity but no survival benefit.79
Following the discovery that hydroxychloroquine can enhance the antitumor effect of cancer therapies by inhibiting autophagy-related stress tolerance in tumor cells,80 an ongoing phase II study is investigating the combination of HD IL-2 and hydroxychloroquine.71 In addition, genistein may arrest tumor cell growth by various mechanisms including inhibition of cell proliferation, induction of apoptosis, induction of differentiation, and modulation of cell cycle progression.81 A current phase II trial is investigating the combination of genistein and HD IL-2.72
Immune Checkpoint Inhibition
An enhanced understanding of tumor–host interactions has prompted novel immunotherapeutic strategies for mRCC, and immune checkpoint inhibition has emerged as a potential therapeutic strategy. The lymphocyte protein receptor programmed death-1 is an inhibitory checkpoint receptor that plays a major role in T cell activation (see Figure 2).82 Levels of immune cells expressing PD-1 are increased in patients with high-risk RCC tumors.84 Anti-PD-1 monoclonal antibodies may therefore control tumor growth and are in active clinical development. Phase I clinical studies have demonstrated antitumor activity for the human monoclonal antibody directed against PD-1, nivolumab (previously known as BMS- 936558),85,86 and durable responses have been observed in mRCC.87 Several ongoing trials are further investigating the use of nivolumab in RCC. A phase I trial is incorporating pretreatment and post-treatment biopsies in an attempt to define biomarkers predictive of response. This trial also includes a cohort of previously untreated patients.88 Another phase I study is investigating the combination of PD-1 blockade with pazopanib, sunitinib, or ipilimumab.89 A potentially pivotal, randomized phase III study is comparing nivolumab second-line therapy with everolimus for patients with advanced RCC. The primary endpoint of the study is OS.10 Furthermore, nivolumab may potentiate other therapies: a case has recently been reported of a patient with mRCC who experienced no tumor regression following PD-1 blockade in an investigational trial, but subsequently achieved near-complete response to bolus HD IL-2 therapy, and maintained a persistent response off therapy.90 This case emphasizes the need to optimize sequential immunotherapeutic strategies.
Denileukin diftitox (DD) is a fusion protein of diphtheria toxin and human IL-2 that depletes cells expressing the CD25 component of the IL-2 receptor, the alpha chain of the high affinity trimer receptor (CD25, CD122, CD132). In a phase I study, the combination of coordinated DD and then HD IL-2 achieved responses in three of nine patients, with one complete response and no unusual AEs.91
Another potential approach is inhibition of the cytotoxic T-lymphocyteassociated antigen 4 (CTLA4). Antibodies directed against CTLA4 block the interaction between B7 ligands on APCs and CTLA4 on CD8+ and CD4+ T cells (see Figure 2).83 The anti-CTLA4 antibody ipilimumab showed durable partial responses in a phase II clinical study, but was associated with severe toxicities in one-third of patients.92 An association between immune-related toxicity and responses was observed. Combination with IL-2 did not appear to be synergistic, although responses were observed.93 Single-agent CTLA4 inhibitors are not currently an area of active clinical investigation in mRCC. A phase I study assessing the combination of the anti-CTLA-4 antibody tremelimumab plus sunitinib resulted in severe renal toxicity.94
The soluble lymphocyte-activation gene-3 (LAG-3) protein is an agonist of major histocompatibility complex (MHC) class II-driven DC activation that enhances the expansion of tumor-specific CTLs in vitro.95 In a phase I, trial of patients with advanced RCC, IMP321, a soluble LAG-3 fusion protein, demonstrated clinical activity without significant toxicity.74
Targeted immunotherapeutic approached are also in clinical development. The immunotoxin naptumomab estafenatox (Nap) was developed in an effort to activate and target the patients’ own T cells to their tumor, by fusing a superantigen variant that activates T lymphocytes to the Fab moiety of a tumor-reactive monoclonal antibody. However, a recent phase II/III trial of Nap + IFN-a did not meet its primary endpoint.96
Vaccine Immunotherapies
Priming of the immune system can enhance the recognition of tumor antigens and increase the chances of an immune response. However, the investigation of vaccine therapy in mRCC has had mixed success. In a phase II study, patients received cytokine therapy or cytokine therapy plus the vaccine TG4010, which targets the glycoprotein mucin-1 (MUC- 1). The vaccine was well tolerated, but conferred no significant OS benefit compared with those treated with cytokines alone.97
IMA901 is a synthetic vaccine comprising 10 different tumor-associated peptides (TUMAPs) that are naturally present in human cancer tissue. A phase II trial showed that a single dose of cyclophosphamide reduced the number of Treg cells and confirmed that immune responses to multiple TUMAPs were associated with longer OS.98,99 The vaccine has now entered a phase III trial comparing treatment with sunitinib to treatment with sunitinib plus a schedule of cyclophosphamide, granulocyte-macrophage colonystimulating factor (GM-CSF), and peptide vaccination, limited to patients with human leukocyte antigen A2 (HLA-A2). It has completed accrual.100
DC vaccines have been extensively researched, the most notable being sipuleucel-T, which received FDA approval for use in prostate cancer.101 DCs are essential for the development of adaptive immune responses, and antigen-loaded DC vaccines have been found to stimulate tumor-specific T-cell expansion in mRCC patients.102 A systematic review and meta-analysis found an objective response rate of 12.7 % in RCC. The combined percentages of objective responses and stable diseases amounted to a clinical benefit rate of 48 %.25 DC vaccines have shown clinical activity in combination with HD IL-2, warranting further exploration of this approach.103,104 Certain targeted therapies, such as sunitinib, may reduce tumor-induced immunosuppression and enhance the tumor microenvironment to promote synergy with autologous DC vaccines.
In a single-arm phase II study (n=21), AGS-003, an autologous amplified tumor RNA-loaded DC–based immunotherapy was administered in combination with sunitinib to patients with poor and intermediate risk factors. Treatment consisted of standard 6-week cycles of sunitinib plus AGS-003 (once every 3 weeks x 5 doses, then every 12 weeks). The median overall PFS was 11.2 months and the final median OS was 30.2 months. Analysis by baseline Heng risk status, showed that the median PFS was 19.4 months for patients with intermediate risk (n=11) and 5.8 months for poor risk features (n=10) at baseline. The median OS was 39.5 months for patients with intermediate risk and 9.1 months for subjects with poor risk.77 These OS and PFS data were almost double those expected for sunitinib in intermediate- and poor-risk patients.28 A phase III study is ongoing.78
Future research to enhance vaccine responses in mRCC will focus on the use of local immunomodulators to boost the activation of APCs, systemic immunomodulators to suppress Tregs and MDSCs and antigens with greater specificity and immunogenicity, as well as optimal scheduling and dosage of vaccines.75
The main development of IL-2 immunotherapy has been pursued as a single agent, in combination with other cytokines such as interferon,27 and with cellular preparations, either autologous leukocytes16,17 or other vaccine preparations.105 Newer immune therapies are being developed in a context of targeted therapies that have already well-defined, established benefit. In this context, it is natural that the new treatments be tested as partner-drugs added on to those treatments; this type of combination can be seen in clinical trials, in which vaccines are combined with sunitinib,78,98 and in NCT01472081 (Checkmate 016), combining sunitinib and pazopanib with nivolumab.106
Radiation Therapy as Priming for Immune Response
Radiation therapy to the tumor sites may enhance immune response by upregulation of tumor antigens.107–109 Preclinical models have suggested that HD radiation can act as an immunologic booster in patients with RCC. A phase I study therefore investigated the use of stereotactic body radiation therapy followed by HD IL-2. The combination could be administered safely and response rate significantly exceeded that expected from historical data.73 This combination therefore warrants further study, and two singlearm studies are open.110,111
Summary and Concluding Remarks
Immunotherapy remains an important therapeutic option for patients with mRCC. For well-selected patients, HD IL-2 can produce excellent results. Targeted agents produce positive results in large numbers of patients, but do not yield long-term durable responses in the same way. For the appropriate, fit, clear cell kidney cancer metastatic disease patient, a therapeutic goal of a durable complete response is reasonable. To date, the most promising strategy among marketed cytokines is the identification of patients who are likely to respond to HD IL-2. The ‘Select’ trial has demonstrated that targeting patients can increase the proportion of patients that respond to HD IL-2, although the hypothesis about histologic testing by CAIX did not demonstrate utility.
Concurrent with the period of time that HD IL-2 has been a marketed medicine, the segmentation of kidney cancer into clear cell type, papillary type 1 and 2, and other lower frequency subtypes has become better defined. The observation that responders to HD IL-2 therapy were consistently among patients with clear cell type helped to define clear cell type as a major feature for selection of patients for possible HD IL-2 treatment. Comparably, the VEGF-pathway drugs, partly in an effort to treat a more uniform group of patients, were approved with pivotal trials for which clear cell type was a required feature. However, there is no empiric basis to define a blanket generalization that ‘non-clear cell’ types of kidney cancer are ‘not immune responsive,’ although it is true that all, or almost all, contemporary monotherapy HD IL-2 treatment is on patients with clear cell type RCC. Given that checkpoint inhibitors and vaccines are making progress in the setting of cancers not at all treated with HD IL-2, such as nonsmall cell lung cancer, there are reasonable rationale to develop these for treatment of kidney cancers that are not clear cell type. The relatively much lower frequency, however, will undoubtedly mean a slower trial accrual and development process.
Improving long-term outcomes for more patients with mRCC remains a goal for immunotherapies with novel mechanisms of action, and possibly in combination with targeted therapies. Recent insights into the complexity of immune manipulation suggest some novel approaches. Despite the clinical failure in some early trials, several immunologic approaches merit further investigation. It is hoped that the results of several ongoing trials will shape future treatment approaches; in particular, the phase III trial investigating the use of nivolumab plus everolimus is awaited with interest. It is not yet known where these novel strategies will fit into a practical, general RCC treatment paradigm, in terms of VEGF, mTOR, and other immune treatments.
The ultimate objective of curing RCC remains challenging, but one may be hopeful that new immune therapies will make this a reachable goal for many mRCC patients.