Renal cell carcinoma (RCC) accounts for approximately 3 % of adult malignancies and 90–95 % of neoplasms arising from the kidney. It is estimated that, in 2011, 60,920 people in the US will be diagnosed with cancers of the kidney and of the renal pelvis, and 13,120 will die of the disease.1 At the Memorial Sloan-Kettering Cancer Center, a number of clinical characteristics associated with differences in prognosis were used to develop a predictive model in RCC.2,3 They form the basis of the risk stratification criteria set by the National Comprehensive Cancer Network (see Table 1).4 In a report from 1999, the median survival of RCC patients in the intermediate-risk group was 10 months, but ranged from four to 20 months across three prognostic subsets, depending on a number of prognostic factors.5 Conventional clear cell RCC is the most common subtype and accounts for more than 80 % of all RCCs.6
Until recently, systemic treatment options for metastatic RCC (mRCC) were limited. Chemotherapy has limited efficacy and, although interferon alpha (IFN-α) can produce marginal survival benefits, response rates are 5–15 % and most of the responses are partial and short-lived.7 The cytokine interleukin-2 (IL-2), a factor that enhances the proliferation and function of T lymphocytes and natural killer cells, had been considered the mainstay of therapy from its approval by the US Food and Drug Administration (FDA) in 1992 until 2006, but it is effective only in a minority of those treated and thus only used in a small number of patients. More recently, new therapeutic options have emerged, including targeted therapies such as tyrosine kinase inhibitors (TKIs) and the agents that inhibit the mammalian target of rapamycin (mTOR).
There are currently eight approved drug therapies for mRCC. These agents have been shown to improve median progression-free survival (PFS) or median overall survival (OS), although none except IL-2 have demonstrated unmaintained durable responses in randomized trials. However, recent retrospective studies and case reports did identify some long-term survival benefits in patients taking TKIs.8–10
This article will outline the current standard of care in the treatment in mRCC with a perspective on immunotherapy in the era of targeted therapy, and review research into combined and targeted therapies and evolving therapeutic strategies. Additionally, it will discuss further evolution of paradigms, with an emphasis on tailoring therapies and achieving complete response.
Current Treatment Pathways
It is important to be aware that mRCC is a heterogeneous disease and that no single-standard treatment algorithm should be applied to all patients. Initially, when patients present with mRCC, they are evaluated for possible cytoreductive nephrectomy and metastasectomy. If metastatic disease persists following these treatments, then systemic therapy must be considered. Initial treatment involves observation in selected cases, locally directed therapies including resection, or medical treatment. The latter involves one of the approved agents (including cytokines and antiangiogenic targeted therapies – see Table 2) or participation in a clinical trial.
When assessing a patient for treatment, disease histology is first taken into account, clear cell histology being treated differently from non-clear cell histology. Risk is assessed according to a variety of scoring systems, the most frequently used being the Memorial Sloan-Kettering Cancer Center criteria. Among patients in need of medical treatment, those with good-to-intermediate risk cancer and clear cell features are offered antiangiogenic agents, while those who meet the appropriate criteria (see below the section on immunotherapy) are offered high-dose IL-2. For patients who have high-risk disease, treatment options include mTOR inhibitors such as temsirolimus4 or other targeted therapies. For patients with non-clear cell subtypes, targeted therapies still dominate the available options, consistent with the FDA-approved drug label information.
It is important to set goals in terms of stabilization and improvement expected from first-line treatment, and involve the patient in the therapeutic decisions regarding when to move on to the next treatment stage. Choices are wider for second-line treatment and beyond, any of the targeted agents being possible options. Everolimus and axitinib have demonstrated efficacy in randomized trials that were specifically highlighting the population with prior TKI treatment targeting the vascular endothelial growth factor (VEGF) pathway.11,12 It is plain that there are potentially multiple lines of therapy, and the choice can be daunting.
Immunotherapy
When considering a new paradigm in the treatment of mRCC, the focus should be on optimizing cure fraction – i.e., complete response and remission. The rise in popularity of targeted agents has resulted in a decline in the use of immunotherapy, such as high-dose IL-2, because it is effective only in a minority of patients and is associated with severe acute toxicities.13,14 The new agents provide benefits in terms of PFS; however, high-dose IL-2 remains the agent with proven efficacy in producing complete remission in patients with mRCC.
In 1992, high-dose IL-2 was approved by the FDA for the treatment of patients with mRCC based on data from seven Phase II trials.15 Following this, a randomized trial compared low-dose and high-dose IL-2 in mRCC. The response rates in patients treated with high-dose IL-2 were twice as high as those seen in patients treated with low-dose IL-2 or subcutaneous IL-2 alone. In addition, many more durable complete responses were seen in the high-dose IL-2 arms, many of these responses lasting more than a decade in duration.16 A subsequent Phase III trial (n=192) found that high-dose IL-2 induced a response rate of 23.2 % and a median survival of 17.5 months in patients with mRCC, and concluded that it should remain the preferred treatment in selected patients.17
Given that high-dose IL-2 is currently the only approved therapy that can induce such durable complete responses, it should be considered more often as first-line therapy. However, it is currently not possible to identify patients who will benefit from it. The importance of assessing patient suitability is illustrated in a recent case series of 72 patients with RCC given high-dose IL-2 as first-line treatment. The overall response rate, irrespective of histology, was 27 %; however, following the stratification of patients according to histology, the response rate was 52 % in the subgroup of patients with favorable histologic features. Among all patients with a favorable histology, including those identified retrospectively, the combined response rate was 49 %, 25 % achieved complete remission and responses appeared to be durable.18
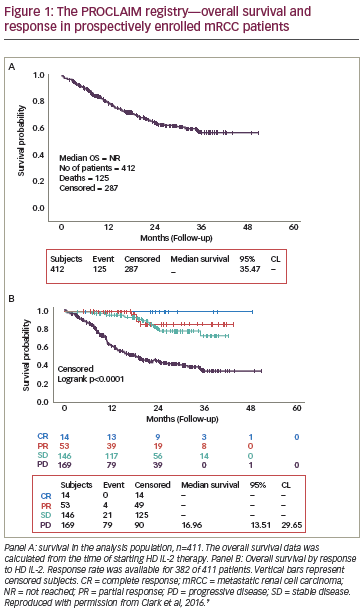
The ideal candidate for treatment with high-dose IL-2 would be relatively young (<70 years), be physically and medically fit, have a good performance status,15 not have any comorbidities or extensive metastatic disease,19 have clear cell histology, and have had nephrectomy more than six months before starting therapy.20 Treatment with high-dose IL-2 should be avoided in patients with severe cardiac, lung, or kidney dysfunction.21 Surprisingly, a Phase III trial comparing high-dose IL-2 with subcutaneous IL-2 plus IFN-α found a significant survival benefit with high-dose IL-2 in a subset of patients with liver and bone metastases (p=0.001) as well as in patients with primary tumors in place (p=0.04) (see Figure 1).17 However, these individuals still did poorly compared with the overall study population, and are unlikely to form a major group from which to identify patients who would benefit from high-dose IL-2. Histology studies have found the greatest response to IL-2 in clear cell histology patients with more than 50 % alveolar and no granular or papillary features.22,23 A lack of retroperitoneal lymphadenopathy has also emerged as a prognostic indicator of survival in IL-2 therapy.24
A biomarker with the ability to predict which patients would benefit from IL-2 before any treatment is initiated would be valuable for maximizing the advantage of this therapy, identifying those with better response potential and directing those with less chance to benefit to other initial treatments. However, finding such a biomarker has been elusive. C-reactive protein, an acute-phase protein synthesized as part of the systemic inflammatory response, showed promise as a potential biomarker in early studies,25,26 but has not, to date, been found to be of clinical use. Carbonic anhydrase IX (CAIX) is a transmembrane member of the carbonic anhydrase family and is thought to play a role in cell proliferation in response to hypoxic conditions. CAIX is not expressed in healthy renal tissue but is expressed in most clear cell RCCs, and was evaluated as a potential biomarker of response to IL-2.27 However, recent results from a non-randomized Phase II trial found a response rate to IL-2 of 30 % in patients with clear cell RCC but did not find significant associations between the response to IL-2 and CAIX.28 A single nucleotide polymorphism (SNP) was identified in the coding region of the CAIX gene and studies to investigate its potential as a biomarker are underway.29
The limitation in evaluating single markers is that the response of a tumor to IL-2 depends on the interplay between two factors: the susceptibility of the tumor to immune attack and the competence of the patient’s immune system. Immune competence can be affected by comorbidities, age, and other physical factors, but also by cytokines made by the tumor, which in turn have a downstream impact on immune function. It is likely that markers of both immune function and tumor features will form part of a comprehensive biomarker approach.
Another consideration related to IL-2 treatment is the fact that some people are not able to take time off work to receive a treatment that requires hospitalization. However, the short-term loss of revenue should be balanced against the long-term health gain – if IL-2 therapy was to be successful, the person would not need lifelong therapy.
Targeted Therapies
As a result of the greater understanding of the molecular mechanisms underlying RCC angiogenesis and growth, several new agents targeting relevant cellular signaling pathways were developed in the last decade (see Figure 2). Several have demonstrated significant efficacy in the treatment of mRCC (see Table 3) in subpopulations much less narrowly defined than for IL-2 treatment, and these medications are the core of current treatment algorithms.
The oral multikinase inhibitors inhibit signaling by multiple targets including vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors. Sorafenib was the first oral multikinase inhibitor approved for use in mRCC.30,31 Sorafenib is a commonly used agent for second-line treatment of mRCC after immunotherapy failure.32 Sunitinib was approved in 2006 and since then has become widely used for the first-line treatment of mRCC; a retrospective analysis showed that patients treated with first-line sunitinib experienced a doubling in OS compared with those treated with IFN.33
Bevacizumab is an intravenously administered recombinant monoclonal neutralizing antibody that binds and neutralizes circulating VEGF. It can be given in combination with subcutaneously injected IFN-α. This combination is indicated for the first-line treatment of patients with advanced RCC or mRCC,34,35 based on two trials that demonstrated an improvement in PFS with bevacizumab plus IFN-α versus IFN-α alone.
Pazopanib was approved in 2009 following a Phase III study in which it doubled the median time to PFS compared with placebo.36 Axitinib is the most recently approved antiangiogenic agent, demonstrating an improvement in PFS when used as a second-line treatment compared with sorafenib (OS was not statistically different between the two arms).12 Other therapeutic agents target mTOR, which is a serine/threonine kinase and a central component of the signaling pathways governing cell proliferation, survival, metabolism, angiogenesis, and apoptosis. The FDA has approved two mTOR inhibitors for kidney cancer therapy. Temsirolimus induced an improvement in OS in mRCC patients who had features indicative of a poor prognosis37 and is a recommended first-line treatment in such patients.4 Everolimus is indicated for use following failure of treatment with sorafenib or sunitinib.11,38 Clinical trials are now investigating the efficacy and safety of everolimus in the first-line setting, among other sequencing strategies.
The advantage of targeted therapies is evident: unlike what is seen with cytokine therapy, a majority of patients experience clinical benefit. Targeted therapies are associated with improvements in health-related quality of life compared with IFN-α treatment.39
However, unlike high-dose IL-2, which can achieve a durable complete response, that type of response has not been seen in the randomized trials investigating targeted therapies, but only described in case reports.8–10
The selection of a targeted agent in mRCC should take into account disease status, histology, and patient status. In symptomatic patients and in patients with large tumor burden, an agent such as sunitinib is indicated because of the rapid tumor shrinkage often observed with this drug. In patients who have multiple (more than two) prognostic risk factors, temsirolimus is preferred, considering that the pivotal temsirolimus trial demonstrated a survival benefit in this specific patient group. Sorafenib and everolimus have been reported to be well tolerated in elderly patients in first- and second-line settings.40 In asymptomatic patients, especially those with predominantly lung metastases, bevacizumab plus IFN-α is an option, because it has been observed that a benefit from IFN-α is more likely in this patient population.41 However, current guidelines have fundamental limitations when applied to patients from the general, non-study population: treatments for mRCC have not usually been evaluated specifically for patients with comorbidities and/or who are receiving several medications.42
Studies are in progress to find predictive biomarkers of response to targeted therapies, but to date few are published. Clinical features may also have predictive value: the development of sunitinib-associated hypertension has been correlated to a favorable tumor response to this drug.43,44 Molecular studies of tumor cells may help direct future research: in a small retrospective study, loss-of-function mutations in the von Hippel-Lindau (VHL) gene appear to loosely correlate with response to VEGF-targeted therapy.45 Additional work is needed to better define the relationship between molecular features and response to therapy. In addition, several investigators have pursued the identification of SNPs in genes associated with the actions of targeted therapies.46,47 Circulating factors may also provide insights into determinants of response and resistance.48 A coordinated effort is required to bring these assays to the point of testing in prospective trials.
While the success of targeted therapies established a new standard of care in the treatment of mRCC, there remains a profound need for new agents or combinations of treatments that will improve clinical activity while carrying a reduced risk of treatment-associated toxicities. With the current agents, side effects are frequent – although virtually all can be managed effectively.49 The area of targeted therapies is rapidly changing, as new agents are introduced, supported by evidence from randomized controlled trials; however, few data directly compare newer versus more established agents or specific drug selection strategies.
Combining and Sequencing Therapies
An active area of current research is to determine better combinations and sequencing of therapies. Combination therapy has the potential for additive or synergistic effects as a result of a more complete blockade of underlying molecular signaling pathways. Further, combination therapy has the theoretical potential to delay the development of resistance, compared with single-agent therapy that may induce compensatory mechanisms. While around 40 % of patients receiving sunitinib for the treatment of mRCC show an initial partial response as defined by the Response Evaluation Criteria In Solid Tumors (RECIST), the majority develop resistance and progressive disease after one year. 50 Combining treatments presents an attractive way of maximizing tumor response, but any efficacy gains must be balanced against the possibility of greater toxicity.
Increased toxicities remain a barrier to the success of combinations of targeted therapies.51 The combination of sorafenib and bevacizumab was evaluated in a Phase I trial and resulted in an unexpected level of toxicity at lower doses.52 A Phase I trial of sunitinib plus temsirolimus was terminated due to significant toxicity observed at low starting doses of both drugs.53 A Phase I trial of tivozanib plus temsirolimus found that the combination was tolerable at standard doses of both agents, but the relative response rate and durability were not evaluated.54
Immunotherapy may play a role in combined therapeutic approaches. Several combinations of IL-2, IFN-α, and other chemotherapeutic agents such as 5-fluorouracil have been tried over the years.55 These have, in some cases, resulted in apparent improvements in outcomes, but have not been shown in a randomized setting to give clinical benefits that would outweigh the greater levels of toxicity. In a Phase III trial, the combination of subcutaneous IL-2 and IFN-α was not more effective than high-dose IL-2 alone.17 In another open-label randomized trial, the combination of IFN-α and IL-2 did not improve OS or PFS compared with IFN-α alone, but did induce long remissions in a small number of patients. No significant increase in toxicity was observed with this combination.56 Results from a Phase II trial incorporating bevacizumab with low-dose IL-2 suggest that this combination has modest clinical activity in mRCC and does not confer additional toxicity.57 Sorafenib plus low-dose IFN-α was found to be active and well tolerated in several Phase II trials;58,59 however, the addition of low-dose IFN did not appear to improve response rate or PFS in a randomized study.59 In addition, recently published data from a Phase II trial found that the combination of sorafenib and IL-2 did not demonstrate improved efficacy compared with sorafenib alone.60
Given the toxicity issues associated with combination therapies, sequential therapy remains the standard of care and current research aims to determine an optimum sequence of targeted agents. Currently, the only agents with specific, Phase III trial-based recommendations for use after primary VEGF-targeting TKI therapy are everolimus and axitinib,4 although any of the targeted agents can be considered after cytokine therapy. Several clinical trials are underway to evaluate the optimal combination and sequencing of targeted therapies; these trials are summarized in Figure 3. A recent review concluded that the optimal sequence may vary between patients according to comorbid conditions and/or disease stage.61
Cytoreductive Nephrectomy
Cytoreductive nephrectomy has been shown to improve OS in mRCC patients.62 Following the introduction of targeted therapies, it is unclear to what extent, when such therapies are used, there is still as consistently a benefit from cytoreductive nephrectomy. Ongoing randomized prospective trials will address this question. However, the majority of patients in the sunitinib and sorafenib Phase III trials underwent nephrectomy as part of their prior treatment30,32 and, in a retrospective study, cytoreductive nephrectomy was independently associated with a prolonged OS in patients with RCC treated with VEGF-targeting agents.63 As a separate issue, regarding symptoms as opposed to survival, cytoreductive nephrectomy may be offered to patients experiencing symptoms related to the primary tumor, such as pain caused by the kidney mass, intractable hematuria, erythrocytosis, uncontrolled hypertension, or persistent hypercalcemia,64 or to individuals with a performance status of 0 or 1 and resectable primary tumor meeting the eligibility criteria of the upfront nephrectomy trials reported by Flanigan et al.62
Emerging Agents and Novel Treatment Approaches
In a Phase III clinical trial of tivozanib, a small molecule that inhibits VEGFR, this targeted agent completed accrual and PFS data were reported. Tivozanib was shown to extend PFS in treatment-naive mRCC patients compared with sorafenib (median PFS of 12.7 months versus 9.1 months, respectively) and is being considered for FDA approval.65
Histone deacetylase (HDAC) inhibitors have antitumor activity in different tumor models including RCC. The combination of IL-2 with the HDAC inhibitor MS-275 has been found to have a synergistic antitumor effect in a murine RCC model.66 Other promising targeted agents for which Phase II clinical trial data are available include regorafenib (BAY 73-4506), a multikinase inhibitor,67 and cabozantinib (XL-184), an inhibitor of the tyrosine kinase c-Met and of VEGFR2.
While most clinical trials focus on patients with mRCC exhibiting clear cell histology, inhibitors of c-Met (a proto-oncogene that encodes a protein know as hepatocyte growth factor receptor [HGFR]), such as GSK1363089 (formerly XL880) and ARQ197, have been developed.68 This reflects the observation that genetic alterations in papillary RCC are different from those seen in clear cell RCC. These agents are at the Phase II clinical trial stage.68,69
Newer immunomodulatory agents are also in development. The inhibitory receptor CTLA-4 regulates tolerance of T cells for both normal and tumor-associated antigens. A Phase II study found that the anti-CTLA-4 antibody ipilimumab induced cancer regression in some patients with clear cell mRCC, even in those who had not responded to other immunotherapies.70 Ipilimumab has been given in combination with IL-2 to treat metastatic melanoma71 and was recently approved by the FDA for metastatic melanoma, following a Phase III trial that was the first to show an increase in OS in this condition.72
Programmed death-1 (PD-1) is an inhibitory receptor expressed on activated T cells. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcomes for patients with RCC.73 Several anti-PD-1 antibodies are being evaluated for the treatment of mRCC74 and Phase II trials are underway. Other novel agents currently under investigation include:
- WX-G250, a monoclonal antibody that binds to CAIX;75
- agents that inhibit tumor-induced immunosuppression, such as transforming growth factor β antibody and purified antihuman CD274 (PDL1) antibody;76
- agents that activate T cells (e.g., CD-137 antibody and IL-21;77 and
- agents that activate dendritic cells (e.g., toll-like receptor agonists).78
Interest in vaccines for mRCC remains high. IMA901 is a therapeutic cancer vaccine based on the selection of naturally presented tumor-associated peptides. A Phase II study found that IMA901 is safe, well tolerated, and immunogenic, and OS compared favorably with currently approved drugs.79 A Phase III trial is underway that looks at sunitinib therapy with or without the addition of a peptide vaccine, granulocyte macrophage colony stimulating factor, and cyclophosphamide pre-treatment.80 The trial is limited to HLA-A*0201 (+) patients because of the design of the peptides, which only associate with HLA cell surface protein. Sunitinib therapy was found to augment measures of T cell immunity by modulating immunosuppressive factors and to enhance the potency of a dendritic cell vaccine in vitro.81 As a result of these findings, several clinical trials of sunitinib in combination with a denditric cell-based vaccine in patients with mRCC were launched, including a Phase II study of the immunotherapeutic AGS-003, consisting of autologous dendritic cells loaded ex vivo with tumor cell RNA.82 Early data show promising results. Combination therapy involving a dendritic cell vaccine and IL-2 has been found to be well tolerated and without major side effects.83 For similar reasons, combinations of sunitinib with checkpoint inhibitors, including anti-CTLA-4 and anti-PD1, may be of therapeutic value.
It is possible that the cure fraction of existing cytokine therapies could be increased if they were to be combined with these novel immunomodulatory agents. Potential studies include combinations of IL-2 and checkpoint inhibitors. These trials need to be properly controlled in order to understand the role of each agent, and performed in the context of robust immune monitoring in order to assess whether changes in T-cell response are achieved, whether these changes are linked to clinical benefit, and whether one can identify subpopulations of patients who should receive these combinations.
Implementation of Tailored Strategies in Treatment Algorithms
The implementation of more tailored strategies in treatment algorithms could potentially improve outcomes for patients with mRCC in two ways. One would be to achieve durable complete responses, and the other would be to have more durability within the targeted drug paradigm. Ongoing research to characterize molecular components of both tumor and host should allow the categorization of patients according to sensitivities to particular agents or combinations/sequences of agents. The ultimate aim would be a relatively simple and non-invasive test to direct the choice of a particular drug, drug combination or drug sequence, but achieving this goal will require continued investment in clinical trials and research. Future clinical trials need to be designed to capture material that permits analysis, as promising tests are developed. Collaborative efforts between industry, academic institutions, and funding agencies will help to set up these clinical trials.
Summary and Concluding Remarks
Targeted therapies have largely overtaken immunotherapy as the standard of care in mRCC, representing a great advance for many patients. Less than 10 years ago, the median survival among patients with mRCC was under a year. New developments in targeted therapies and drug sequencing have significantly increased median survival. As more data regarding predictive biomarkers of response to treatment, new targeted agents, and optimal drug combinations and sequences become identifiable, researchers will move closer to the goal of having tailored strategies that can provide maximum efficacy with manageable toxicity.
However, it is important to remember that a subset of patients shows a complete and durable response to IL-2, and ongoing efforts are being made to identify these individuals. Research in immunotherapy is experiencing a renaissance, and ipilimumab—recently approved for melanoma—and other drugs currently in clinical trials offer promise for mRCC. Future trials need to assess the combinations of existing cytokine and targeted therapies, as well as combinations with novel immunomodulatory agents and vaccines. There is also a need to rally the academic and pharmaceutical industry to support trials that obtain blood and tissue samples for translational research. Through integration of clinical and laboratory expertise, paradigms of kidney cancer therapy will continue to evolve. By matching patients to immune therapy or specific targeted therapy, we may see an increase in the cure fraction of patients with mRCC. ■