Metastatic melanoma (mM) and metastatic renal cell carcinoma (mRCC) are potentially lethal cancers with overall poor prognosis. High dose interleukin-2 (HD IL-2; Proleukin®, Prometheus Laboratories Inc, San Diego, California) was approved by the US Food and Drug Administration (FDA) in 1992 for the treatment of mRCC and in 1998 for the treatment of mM. Approval was based on data from a number of clinical trials demonstrating consistent objective responses, including complete responses (CR) in select patients.1 These responses have proven to be durable for decades. However, the use of HD IL-2 is associated with manageable short-term toxicity and requires hospitalization, utilizing the intervention of specialist teams experienced in HD IL-2 therapy.2 In addition, only a minority (16% in mM and 15% in mRCC) of patients respond to treatment, based on the initial data reported in the package insert.1 The emergence of immune checkpoint inhibitors such as the anti-programmed cell death 1 (PD-1) and programmed death-ligand 1 (PDL-1) agents provides a potentially more convenient immunotherapy option. However, durable efficacy data are still pending for PD-1 targeted regimens.
Recent reports confirm that incorporating HD IL-2 into treatment strategies for mRCC can induce CR: in a National Cancer database study (n=91), four patients (4.5%) had a CR, 10 (11.4%) had a partial response, and 28 (31.8%) had a stable disease.3 A retrospective study of patients with mRCC (n=186) and melanoma (n=314) treated with HD IL-2 between 1997 and 2012 reported complete and partial responses in 12% and 16% of patients with melanoma and 7% and 17% of patients with mRCC, respectively.4 Since overall survival (OS) in patients treated with HD IL-2 may extend to decades, registry studies are useful for assessing long-term response, as well as for generating hypotheses to guide future clinical directions. This editorial will discuss the continued role of HD IL-2 within current therapeutic paradigms, as well as discussing the implications of the PROCLAIMSM (Proleukin Observational Registry to Evaluate the Treatment Patterns and Clinical Response in Malignancy) registry on current clinical practice.
The role of HD IL-2 within current therapeutic paradigms
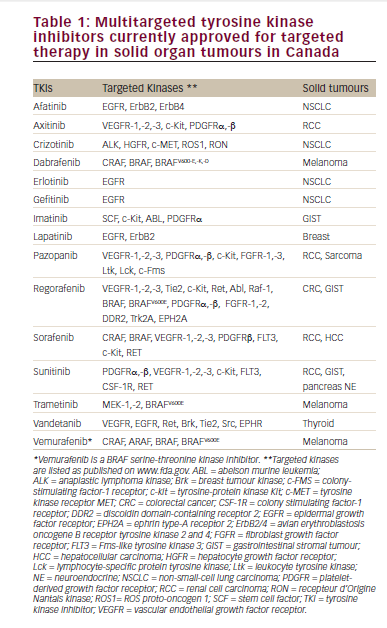
HD IL-2 is an FDA-approved therapy known to produce long-term remissions in mRCC and mM. HD IL-2 was the first approved treatment for mRCC. HD IL-2 outcomes have been better in patients with a good performance status, and and hence it was traditionally used as first line treatment. However, an increasing number of mRCC patients are receiving tyrosine kinase inhibitors (TKIs) or immune checkpoint inhibitors as initial treatment before undergoing HD IL-2 treatment. A retrospective study of 40 consecutive mRCC patients, who were treated with HD IL-2 after at least one prior TKI therapy, concluded that that HD IL-2 was effective and with a manageable toxicity profile in patients who had received prior TKI therapy.5 In melanoma, patients with BRAF mutations in their tumors may receive BRAF/MEK targeted therapy and those with wild-type BRAF status may receive immune checkpoint directed therapy prior to considering HD IL-2.6 The clinical benefit of using HD IL-2 and

immune checkpoint inhibitors in combination or in sequence is relatively unknown and undergoing investigation. The roles of immune checkpoint inhibitors and IL-2 are overlapping but distinct; preclinical data suggest that HD IL-2 and anti-PD1 agents may have synergistic effects.7
The PROCLAIM registry
The PROCLAIM registry is a US-based observational study with over 40 participating sites, and is the largest prospective collection of data from mRCC and mM patients receiving HD IL-2. It was initiated in 2011 with a retrospective cohort (n=370, treated 2007–2012) and then added an ongoing prospective cohort that has enrolled over 1,000 patients to date (www.proclaimregistry.com, NCT01415167). Inclusion criteria were: 18 years of age or older, and to have received at least one dose of HD IL-2. The aims of PROCLAIM are to:
• Collect and analyze information on current IL-2 use.
• Survey the difference in administration approaches for their respective effect on outcomes.
• Evaluate efficacy of IL-2 on response and survival in the treatment of malignant diseases.
• Identify patient- and practice-specific prognostic factors.
• Study the effect of new therapies on IL-2 and efficacy.
• Identify new hypotheses for prospective clinical investigation.
Findings from the registry suggest that the response rates for IL-2 first reported a decade ago are still relevant. Data extracted in 2015 from the retrospective cohort for mRCC patients (n=97) found a median OS of 48.8 months, and an overall response rate (ORR) of 21.5%.8 In the prospective cohort of the registry, HD IL-2 was administered according to the institution’s standard of care, typically involving a 15-minute intravenous infusion every 8 hours at a dose of 600,000 IU/kg or 720,000 IU/kg, with up to 14 consecutive doses over 5 days (1 cycle). A second cycle of HD IL-2 was given after approximately 9 days. Two such cycles constituted 1 course of therapy. Additional courses were administered at the discretion of the treating physician.
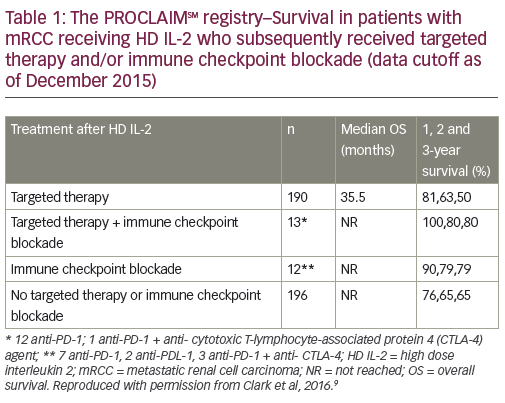
Data from the prospective study involving mRCC patients (median followup 21 months) were recently presented at the 2016 Annual Meeting of the American Society of Clinical Oncology (ASCO).9 Among 382 patients with evaluable data, the ORR was 17.8%, including CR in 3.9%, partial response (PR) in 13.8%, and stable disease (SD) in 38.1%. Among these, the median OS was not reached while in patients with progressive disease (44.2%), the median OS was 17 months (Figure 1). It is noteworthy that OS was not reached in patients with SD. Patients with SD were traditionally considered non-responders, but these data suggest that achievement of SD is a clinically meaningful outcome in response to HD IL-2 therapy. In addition, an analysis was performed of data from mRCC patients who initially received HD IL-2 and subsequently targeted therapy (n=190) and/or immunotherapy (n=12). These results suggest that HD IL-2 therapy followed by targeted therapies and/or immune checkpoint inhibitors may be associated with survival benefit (Table 1).
Another 2016 abstract from the PROCLAIM registry describes survival data from a cohort of patients with mM (n=273, median follow-up 23.1 months).10 The treatment regimen was similar to that described for mRCC. The median OS for all patients (n=273) was 19.4 months. In addition, melanoma patient subgroups were analyzed based on treatment after HD IL-2 as follows: no immune checkpoint blockade following HD IL-2 (n=137), HD IL-2 followed by ipilimumab alone (n=82), and HD IL-2 followed by anti-PD-1/PD-L1 inhibitors with or without ipilimumab (n=54). In the latter group, the sequence of anti- PD-1 and ipilimumab was not fixed. There was no difference in median OS between patients treated with ipilimumab post HD IL-2 compared to patients with no immune checkpoint blockade following HD IL-2 (15.8 versus 14.1 months, p=0.96). However, the median OS in patients treated with anti-PD-1/PDL-1±ipilimumab following HD IL-2 was significantly higher compared with those treated with HD IL-2 alone (28.2 months versus 14.1 months respectively, p=0.002). The estimated 12-month survival rates for no immune checkpoint blockade, HD IL-2 followed by ipilimumab, and HD IL-2 followed by anti-PD-1±ipilimumab were 56%, 64%, and 96% respectively. The authors concluded that there was a good rationale for investigating IL-2 therapy in combination or sequence with anti-PD-1/PDL-1 agents. Such trials are currently being planned.
In a third 2016 abstract, the safety and efficacy outcomes of patients treated with HD IL-2 after PD-1 inhibitors (n=16) were compared with those patients who had not received any immune checkpoint blockade prior to HD IL-2 (n=681).11 Of the former, 12 patients had evaluable data, seven patients with mM also received ipilimumab, and three patients with mRCC also received vascular endothelial growth factor (VEGF) targeted therapy prior to treatment with HD IL-2. The most common toxicities leading to treatment discontinuation during the first cycle of HD IL-2 treatment for patients who had previously been treated with anti-PD-1 agents were: hypotension, diarrhea, vomiting, hypoxia, acute renal failure, and arrhythmia. Similar toxicities were reported among the patients who had not received prior immune checkpoint blockade. No IL-2-related deaths were reported in the cohort receiving prior anti-PD-1. The 12-month survival rates were similar in both groups (76% in patients who previously received anti-PD-1 versus 74% in patients who had not previously been treated with immune checkpoint blockade). The ORR was 8.3% in patients with prior anti-PD-1 agents versus 15% in those without prior immune checkpoint blockade. These data suggest that that HD IL-2 is a treatment option for patients who experience progressive disease after PD-1 inhibition.
Impact of the PROCLAIM data
Data from PROCLAIM to date have provided real-world evidence that can inform current practices and also help the clinician develop new treatment strategies involving newer immune checkpoint inhibitors. The use of checkpoint blockade in everyday clinical practice is currently emerging as a major mode of treatment for patients with mM and mRCC. As we learn more from both clinical trial results and clinical practice experience, it is clear that careful patient selection, clinical monitoring for response and adverse events and rapid intervention for toxicity are needed with these agents. Further, the best practice for integrating checkpoint inhibitors with other available agents, such as IL-2 and antiangiogenic agents, is lacking evidence-based data. Thus, retrospective studies and, perhaps even more importantly, data from the prospective PROCLAIM database have generated new insights and testable hypotheses that are both informing clinical practice and providing avenues for validation in follow-up clinical trials.
The latest findings of the PROCLAIM registry suggest that HD IL-2 is safe and effective before and after targeted therapy and immune checkpoint blockade. Recent data demonstrate survival benefits for patients initially receiving HD IL-2 followed by immune checkpoint blockade, most notably with PD-1/PDL-1 directed agents. Continued analysis of data emerging from PROCLAIM will likely help guide optimal sequencing strategy of HD IL-2, targeted therapies and immunotherapies. Evidence to date support use of HD IL-2 early in the treatment plan. In a series of abstracts, there was no negative affect seen when patients received IL-2 prior to starting checkpoint inhibitors suggesting that first-line IL-2 is safe and allows non-responders to receive subsequent checkpoint blockade without worry that their response may be blunted. There is less data on the role of IL-2 after checkpoint blockade but this will be included in the registry as time progresses. These data also highlight the important issue of combination therapy and suggest that studies of concurrent and sequential approaches may be warranted. In fact, in a recent Phase II study the combination of HD IL-2 and ipilimumab induced a higher CR rate compared with either therapy alone.12