Interleukin 2 (IL-2, Proleukin® Prometheus Laboratories Inc., San Diego, US.) is a recombinant protein that plays an essential role in the development and function of a spectrum of T cells, including regulatory T cells, a naturally occurring population of CD4+ T cells that are vital to the control of inflammatory responses.1 Consequently, IL-2 has numerous antitumor effects, including stimulating T-cell proliferation and function; augmenting natural killer cell proliferation and cytotoxic activity; and triggering the release by activated lymphocytes of cytokines, such as interferon gamma, and tumor necrosis factor (see Figure 1).2
IL-2 was approved by the US Food and Drug Administration (FDA) in 1992 for the treatment of metastatic renal cell carcinoma (mRCC) and was approved for metastatic melanoma (mM) in 1998, after clinical trials demonstrated that it was capable of inducting complete responses (CRs) in a minority of patients.3,4 Following its approval for mRCC, a subsequent clinical trial investigated the use of lower-dose regimens but concluded that superior outcomes were observed with high-dose (HD) IL-2.5 Since then, HD IL-2 has remained one of the few treatments for mRCC and mM with the ability to produce CRs that are durable for decades without further therapy. The administration of HD IL-2 is associated with mainly acute short-term toxicities. One of its most significant toxicities is capillary leak syndrome with effects on multiple organ systems. This results in hypotension and reduced organ perfusion that can be severe but is manageable and reverses upon completion of therapy. The administration of HD IL-2 requires hospitalization with intensive monitoring, in specialized centers with personnel who are experienced in the management of this regimen and its side effects. Thus, not all oncology practices have developed an IL-2 therapy program and referrals to specialized centers have limited the utilization.
The introduction of oral targeted therapies for mRCC has shifted attention from HD IL-2. In the last decade, several targeted therapies have been approved for mRCC, including sorafenib, sunitinib, bevacizumab, pazopanib, axitinib, everolimus, and temsirolimus.6Recently, the tyrosine kinase inhibitor (TKI) cabozantinib has been approved by the FDA following phase III study data that demonstrated improved progression-free survival (PFS), objective response rates, and overall survival (OS) in the second line setting compared with everolimus, in a phase III trial. 7,8 This agent targets MET and KIT in addition to the VEGF receptor. More recently, lenvatinib, an oral TKI of vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor (FGF) receptors 1–4, platelet-derived growth factor receptor α (PDGFRα), RET, and KIT, was approved after it showed improved objective responses, PFS, and OS in combination with everolimus, compared

to everolimus alone in a in a randomized study.9 As with other targeted therapy, chronic toxicities requiring dose reductions were observed with both cabozanatinib and lenvatinib. These therapies require long-term administration, rarely result in CRs, and are associated with the eventual development of resistance. Moreover, the targeted agents result in significant chronic toxicities that need to be prevented and/or managed aggressively throughout ongoing treatment.
Although the use of HD IL-2 has diminished following the introduction of targeted therapies, a recent study (n=2,351) has found a slight increase in use of HD IL-2 in the years 2004 to 2012, possibly reflecting the fact that targeted therapies do not induce CRs and require continuous drug administration.10 In the recent HD aldesleukin ‘select’ trial, in patients with mRCC, 11% of patients remained progression free at 3 years and the median OS was 42.8 months.11 This was achieved utilizing limited drug exposure—2 to 4 weeks in total. The objective response rate of all patients (n=120) to HD IL-2, confirmed by independent review, was 25% (95% confidence interval [CI], 17.5 to 33.7%).12
Recently, a large retrospective study of patients with mRCC treated with HD IL-2 was published. Of 391 patients with mRCC, the best responses on treatment with HD IL-2 were as follows: complete response (CR; 9%), partial response (PR; 10%), stable disease (32%), progressive disease (42%), not evaluable for response (7%). Notably, there were no significant differences in PFS (hazard ratio [HR] 0.74, 95% CI 0.48 -1.1, p=0.14) or OS (HR 0.66, 95% CI 0.39-1.09, p=0.11) between patients achieving PR versus stable disease. However, there were significant differences in PFS (HR 0.13, 95% CI 0.09-0.22, p<0.0001) and OS (HR 0.33, 95% CI 0.23-0.48, p<0.0001) between patients achieving stable disease compared to those with progressive disease and who were not evaluable. In conclusion, a meaningful survival benefit with HD IL-2 was achieved in ~50% mRCC patients and extended beyond to those achieving complete or PR, i.e., to those patients who only achieved stale disease as the best response.12
In very recent years, the programmed death-1 (PD-1) pathway, a regulator of tumor-induced immune suppression, has become therapeutic target in solid tumors. Nivolimumab, a checkpoint inhibitor of binding of PD-1 with its ligands, received FDA approval for mRCC as second-line therapy,13 following a randomized, open-label phase III study (n=821) in which patients with advanced RCC who had received previous treatment were randomized to nivolimumab or everolimus. The OS was longer for the nivolumab group versus the everolimus group, 23.6 months versus 19.8 months respectively and fewer grade 3 or 4 adverse events occurred with nivolumab compared with everolimus.14 However, the CR rate for nivolumab was only 1% and <1% in the everolimus group. In addition, in a phase I study (n=34) nivolimumab demonstrated objective responses that in some patients persisted after drug discontinuation.15 While these responses are promising, treatment with nivolimumab currently requires long-term drug administration and patients do experience side effects such as fatigue, rash, pruritis, nausea, and diarrhea. The durability of response continues to be evaluated.
Treatment options for mM have also expanded in the past several years. In BRAF-mutated mM, approved targeted agents including vemurafenib, trametinib, and dabrafenib, are widely available. In comparison with single agent BRAF inhibitors, the combination of BRAF and MEK inhibitors has shown significant improvement in efficacy outcomes in addition to fewer side effects related to paradoxical activation of the MAPK pathway. The combination has now become the standard of care in patients with BRAFV600 mutated advanced melanoma for whom targeted therapy is used.6
In addition, newer immunotherapy agents, such as ipilimumab, an immune checkpoint inhibitor of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) has been approved for mM, as have nivolumab and pembrolizumab.6 However, no consensus exists on how these therapies should be sequenced, nor how IL-2 should be placed in the sequence.16 Ipilimumab is associated with a median OS of 10.1 months, and an overall response rate (ORR) of 11%.17 Recent phase III study data comparing pembrolizumab with ipilimumab as first-line therapy showed prolonged PFS and OS with less high-grade toxicity in patients with advanced mM; estimated 12-month OS rates were 74.1%, 68.4%, and 58.2% for pembrolizumab given every 2 weeks, every 3 weeks, and for ipilimumab, respectively.18 As a result, the FDA approval of pembrolizumab has recently been expanded to include first-line treatment of patients with unresectable or mM.19 The combination of nivolumab and ipilimumab has also been investigated; in the phase III CheckMate 067 trial, among previously untreated patients with mM, nivolumab alone or combined with ipilimumab resulted in significantly longer PFS than ipilimumab alone.20 Doses and schedules of the combinations are still being evaluated. In the recent phase II CheckMate-069 trial, the CR rate for the combination of ipilimumab and nivolimumab was 22% versus none in the ipilimumab monotherapy group for patients with BRAF wild-type mM.21 The PFS for the nivolumab/ipilimumab treated group has not been reached but was 3.0 months for ipilimumab alone. As a result, the FDA approved the combination of nivolumab and ipilimumab for the treatment of patients with BRAF V600 wild-type, unresectable, or metastatic mM.M22 However, there is significant toxicity with the combination, with 54% of patients on nivolumab/ipilimumab reporting grade 3/4 toxicities and 24% of patients receiving ipilimumab alone. These new data do not support using ipilimumab as single agent in the first-line setting.
While targeted therapy has become more prominent as first-line therapy for mRCC and mM, due in part to ease of administration,6, and important advances have been made in the use of immune checkpoint inhibitors, some studies suggest that HD IL-2 may be the best first-choice therapy.23–25 This review aims to summarize the current status of HD IL-2 immunotherapy in mRCC and mM, to describe recent registry and single institution data on HD IL-2 efficacy and safety, to examine the future challenges of HD IL-2 therapy, and to outline strategies aimed at enhancing the response of IL-2 in combination with other immunologic approaches.
The role of interleukin-2 in the era of targeted therapy—real world data
PROCLAIM data base registry
While phase III clinical trials of new agents are essential for regulatory approval, registries are hypothesis generating and collect data based on real-world use of these agents over a long period of time. This is particularly useful in assessing response to HD IL-2, where survival may extend to decades. In addition, registries enable a better understanding of current clinical trends with respect to particular agents. The Proleukin Observational Registry to Evaluate the Treatment Patterns and Clinical Response in Malignancy (PROCLAIM) registry, a US-based HD IL-2 observational database with over 40 participating sites, consists of a retrospective cohort (treated 2007 to 2012) and an ongoing prospective cohort (over 1,300 patients as of 2015, with enrollment starting September 2011).26 The goals of the registry are to: provide real-world information regarding use of HD IL-2; compare the difference in administration approaches in terms of effect on outcomes; validate efficacy of HD IL-2 on response and survival; identify patients and site-specific prognostic factors; and study and potentially guide the emergence of new therapeutic options.26 Several reports have already been generated from both the prospective and retrospective data.27–31
Single institution registries
Other single center retrospective studies support the continued use of HD IL-2 in the treatment of mRCC and mM. A recent report evaluated the outcomes of 500 patients with mRCC (n=186) or mM (n=314) treated with HD IL-2 between 1997 and 2012 from Providence Medical Center in Portland, OR. The ORR in mM was 28% (CR 12% and PR 16%), and in mRCC was 24% (CR 7% and PR 17%). The 1-, 2- and 3-year survival were 59%, 41%, and 31% for mM, and 75%, 56% and 44%, for mRCC, respectively.24 The total number of patients in this retrospective study is greater than other IL-2 studies from either single or multi-institutions. These observations are comparable to findings from other T cell directed antibody monotherapy studies.
In addition, evidence is emerging to suggest that HD IL-2 should be the first-line therapeutic option in mRCC, with targeted therapies reserved for salvage therapy. In a retrospective nonrandomized cohort (n=286) of patients with mRCC treated from 2003 to 2010, 21 patients treated with salvage-targeted therapy after progressing on HD IL-2 experienced a significantly greater disease-specific survival (median not reached) than 109 patients treated with targeted therapy alone (30 months; p=0.004).25 These findings were supported by a retrospective analysis (n=906) that showed that 43 patients receiving HD IL-2 followed by targeted therapy had the best median OS (40 months). A retrospective analysis of a kidney cancer database that analysed median OS found that patients were less likely to respond to HD IL-2 if they had received other forms of treatment first.23
In summary, HD IL-2 continues to play an important role in the treatment of mM and mRCC. In orderto optimize the role of IL-2, further studies in sequence and combination with targeted therapy and immune therapy are required. Prospective trials are currently ongoing.
The challenges of employing HD IL-2 therapy Logistics
HD IL-2 therapy requires an experienced treatment team, with knowledge of appropriate patient screening and selection, as well as of treatment-related toxicity management. A recent expert consensus document concluded that physician education in the administration of HD IL-2 and management of patients on treatment can increase the number of patients who might benefit from the therapy, and also stated that peri-treatment-related mortality can be reduced to less than 1% when management and selection guidelines are diligently followed.33 Additionally, community oncologists need to be aware of the available therapies for mRCC and metastatic malignant melanoma (mMM) and be willing and able to refer patients for HD IL-2. Many of the challenges associated with the use of HD IL-2 result from knowing which patient is an appropriate candidate for the therapy, and finding a referral center experienced in HD IL-2 patient management. However, the process is similar to that required for screening and referral of patients for SCT. Subsequently, patients return to the primary oncologist, and often are jointly followed.
Biology
Ongoing clarifications of understanding of the immune system have demonstrated complex checks and balances for control and limitation of immune responses. These include checkpoints to control or turn off activation, as well as activation of regulatory T-cells (Tregs) and myeloidderived suppressor cells, which modulate/reduce the immune response. These processes maintain homeostasis. However, they can also inhibit anti-tumor immune responses. A study of eight mM and mRCC patients found that HD IL-2 treatment resulted in increased levels of Tregs.29 This has been confirmed by other studies,35,36 and may be one of the factors that limits the efficacy of HD IL-2: activated Tregs may hinder the generation of antitumor immune responses. Selective inhibition of HD IL-2-mediated enhancement of Tregs may improve the therapeutic effectiveness of HD IL-2 administration, but this is difficult to achieve in vivo. Differences in the ongoing immune response such as the ratio of effector T cells to Tregs may impact the response to HD IL-2 treatment. In contrast, another study in mM patients found that patients with higher levels of Tregs may predict benefit from HD IL-2.35 Thus, there is still a need to better understand the effects of IL-2 on the components of the immune system and ways that these effects may be enhanced for better therapeutic outcomes.
Biomarkers of response
There is a need for biomarkers, such as genetic polymorphisms or the expression of tissue antigens or serum proteins, which may enable identification of patients that would benefit from HD IL-2 treatment. While a small study has identified potential biomarkers including serum VEGF and fibronectin,37 these have not been explored in larger studies. One report associated high numbers of CD8+ T cell infiltrates within mM with prolonged survival.38 It is not yet clear whether the most informative biomarkers will be tumor specific, or host immune system specific. Again, this requires prospective validation.
Clinical predictors in metastatic renal cell carcinoma and metastatic malignant melanoma
In terms of mRCC, the HD aldesleukin “Select” trial prospectively evaluated whether the ORR of patients with mRCC with good predictive pathologic features based on a selection model (including clear-cell histology sub classification and carbonic anhydrase-9 immunohistochemistry staining), was significantly higher than the ORR of a historical, unselected population.10,39,40 While HD IL-2 produced durable CRs and prolonged survival in both “good” and “poor-risk” patients, the model did not improve patient selection criteria. Novel markers (e.g., tumor PD ligand-1 [PDL-1] expression) appear useful in mRCC, but require further study.39,41 A small (n=120), retrospective analysis of tissue obtained from the patients enrolled in the “Select” trial in mRCC suggested that PDL-1 and PDL-3 tissue expression may predict for better response to IL-2.41 In the PROCLAIM registry, ECOG performance status and normal LDH were identified as helpful in predicting the response of patients with mRCC to HD IL-2.42
In 1995, Fyfe et al. reported that three factors predicted for improved survival: Eastern Cooperative Oncology Group (ECOG) performance status, prior nephrectomy, and time from diagnosis to treatment. Baseline ECOG performance status was the only symptom predictive of response.3 Clinical screening to date continues to be based on physiology and histologic subtype as we learn more from the prospective “Select” trial.39
Atkins et al. found that ECOG performance status and prior systemic therapy were only predictors of response in patients with mMM.4 Additionally, a retrospective study of 305 mM patients who received HD IL-2 monotherapy failed to identify any pretreatment factors that were strongly associated with increased ORR or long-term survival.43 In the PROCLAIM registry study, LDH and clinical response were independent predictors of survival in mM patients, similar to several other clinical studies.27 The melanoma IL-2 SELECT trail is ongoing, with clinical and biological correlates being evaluated.44
Additionally, in a recent study, of 50 consecutive mM or RCC patients treated at a single center, the clinical benefit group developed autoimmune phenomena (mM patients) and had a lower platelet nadir, and received the same number of IL-2 doses as the no clinical benefit group, suggesting that clinical response is related to the patient’s baseline immune status and providing baseline information for future prospective analysis.45
In summary, identifying responders to HD IL-2 beyond clinical features and selection of appropriate candidates remains a substantial unmet need and further study is required.
Approaches may enhance clinical benefits of interleukin-2
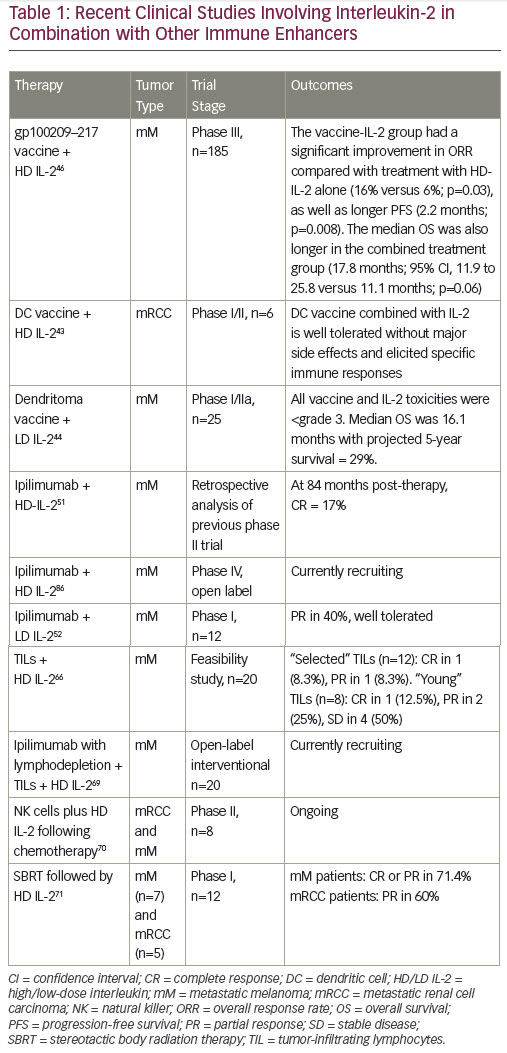
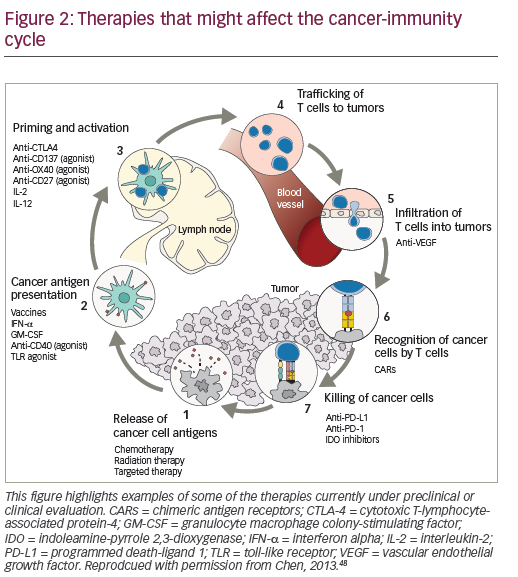
Combined therapeutic approaches provide an opportunity to enhance the response to HD IL-2.46,47 Current options include combinations with targeted therapies or with the new immune therapy approaches or with activated cellular approaches. Reversal of immunosuppression in the microenvironment of the tumor is another focus of the study. Multiple immunosuppressive mechanisms combine to inhibit antitumor immune responses, providing a rationale for the use of combinations of immunotherapeutic agents with different mechanisms of action, with the aim of eliciting synergistic effects to further enhance immune responses against mRCC and mM. Preclinical data support potential synergy between IL-2 and PD-1/PD-L1 pathway inhibition, and this is generating considerable enthusiasm for further investigation.48,49 Clinical trials are planned and ongoing to evaluate the role of IL-2 in combination and sequence with other immune therapies.
The recently approved checkpoint inhibitors are key agents for exploring combination immunotherapy.50-52 (Figure 2). While the combination of IL-2 and nivolumab has not been investigated, in a recent case report, a patient with mRCC with no tumor regression following nivolumab treatment (given on an investigational trial), subsequently achieved near-complete durable response to HD IL-2 treatment.53
A study examining the effects of co-administration of HD IL-2 and ipilimumab in mM patients showed that the combination had an increased CR rate compared with either HD IL-2 or ipilimumab alone. A subsequent evaluation of patients receiving both therapies showed that 17% had a CR at a median follow up of 84 months post therapy.52 Several ongoing trials are investigating the combination of checkpoint inhibitors with HD IL-2.54,55
Vaccines
Vaccines that stimulate anti-tumor immune responses by enhancing antigen presentation have for many years been an active area of research in mRCC and mM. In vitro studies demonstrated that vaccination with the peptide gp100 resulted in high levels of circulating T cells capable of recognizing and killing mM cells,56 leading to the hypothesis that the combination of a vaccine and HD IL-2 may have a synergistic effect on T cell activation. Many approaches involving co-administration of a variety of types of vaccines to improve antigenicity with HD IL-2 to stimulate effector cells are currently being investigated for mRCC and mM. A phase I/II trial (n=10, of which 6 were RCC patients) concluded that a dendritic cell (DC) vaccine combined with low dose (200 MIU) IL-2 was well tolerated in patients without major side effects. Stable disease was reported in one RCC patient. DC vaccine-related antigen-specific immune responses were elicited in six patients, but these did not correlate with clinical responses.57 Data from a recent phase I/IIa study in mM indicate that an autologous tumor lysate-DC fusion (dendritoma) vaccine is effective in combination with LD IL-2.58 Following the promising outcome of a phase II trial,59 a phase III trial investigated a gp100209–217 analog peptide vaccine combined with HD IL-2 in patients with stage II/IV mM. The median OS was also longer in the combined treatment group (17.8 months; p=0.06).60 In addition, a recent retrospective study found that patients who received HD IL-2 followed by a patient-specific mM stem cell vaccine had better survival than those receiving HD IL-2 alone.61
Cell therapy
Adoptive cell therapy (ACT), activating and utilizing tumor infiltrating lymphocytes (TILs) in combination with IL-2, can induce CR and durable responses in mM patients.62 This therapy involves the isolation of TILs grown from a patient’s tumor and expansion of these autologous TILs prior to their reintroduction into a patient. In addition, lymphodepletion prior to TIL infusion may improve the tumor microenvironment by reducing competition for growth factors and cytokines. A study found that lymphodepleting chemotherapy followed by the transfer of highly avid antitumor lymphocytes resulted in significant tumor regression in patients with IL-2 refractory mM.63 Another study compared minimally cultured or selected autologous TILs together with HD IL-2 following lymphodepleting chemotherapy in mM patients. The patient cohort receiving minimally cultured cells showed encouraging responses and minimal HD IL-2 toxicity.64 A subsequent phase II study (n=20) showed that lymphodepleting chemotherapy followed by transfer of short-term cultured TILs can mediate tumor regression in 50% of mM patients with manageable toxicity.65 Several ongoing trials are investigating the combination of ACT with both HD IL-2.54,56 The combination of ACT and low-dose IL-2 has also been investigated, but to a lesser extent. A pilot study concluded that this approach may induce complete and durable
responses while significantly decreasing toxicity in mM.61,66 However, it is not suitable for all mM patients due to technical issues: the metastatic nodule must be at least 2 cm in diameter and only half of the resections grow cells suitable for infusion. Current research is investigating simpler and faster methods to grow TILs.67
Radiation as an antigen-presenting mechanism
Preclinical models have suggested that HD radiation may act as an immunological booster in patients with mRCC.68 A phase I study (n=12) explored the safety of and tumor response to stereotactic body radiation therapy (SBRT) followed by HD IL-2 in patients with mM (n=7) or mRCC (n=5). A CR or PR was seen in eight of the patients (66.6%, one CR and seven PR). In addition, six of the patients with PR as assessed by computed tomography (CT) had a CR when assessed by positron emission tomography (PET) imaging. Among mM patients five of seven of patients had a PR or CR, and three of five patients with RCC had a PR. The response rate significantly exceeded that expected from historical data, and the combination was safe.69 This is being investigated in ongoing studies.70
Intralesional therapy
MM is a very heterogeneous tumor, and intralesional therapy has the advantage of utilizing the patient’s own tumor as a source for tumorspecific antigens. In order to reduce systemic toxicity and increase local therapeutic effects, intralesional treatment may be a promising option in mM; a number of phase II studies have found that intralesional administration of HD IL-2 elicited complete local responses in a high proportion of patients.71–74 A phase I study found that intratumoral injection of IL-2 in combination with ipilimumab expanded TILs and activated a systemic immune response in the majority of patients.75 Treatments were well tolerated. The only grade 3 toxicity observed was injection/tumor site ulceration/necrosis, not a dose-limiting toxicity per protocol. Other toxicities were grade 1 in nature. An abscopal effect (response in at least 1 non-injected lesion) was seen in 9/12 patients.75 An exploratory study suggests that intralesional therapy with HD IL-2 and another cytokine, low-dose granulocyte-macrophage colony-stimulating factor (GM-CSF) in metastatic and primary cutaneous mM may be a promising approach.76
Despite these promising findings, this strategy of targeting local expression of cytokines in tumors will be difficult extend to the treatment of metastatic disease, where disseminated tumors are often inaccessible. It has been postulated that treating a small number of accessible sites may initiate a vigorous systemic immune response that would result in a response in untreated tumor sites. However, studies to date suggest that the treated tumor site is almost always more affected than the distal untreated tumor sites.77 This suggests that the therapeutic efficacy depends not only on the generation of an immune response but that also the alteration of the cytokine tumor microenvironment, an area of active research.78,79
Variants of interleukin-2
Another approach aims to develop variants of IL-2 that exhibit enhanced binding to the IL-2 receptor. An example is EMD 521873 (Selectikine), a fusion protein combining high affinity binding to the IL-2 receptor with an antibody targeting DNA. This agent is in clinical development and has shown efficacy as monotherapy in mM83 and in sequential combination with radiotherapy in metastatic non-small cell lung cancer following first-line chemotherapy.84 A phase IIa study of this agent in combination with SBRT in mM is ongoing.85 Other IL-2 variants are in early development.
Summary and concluding remarks
As therapeutic options for mRCC and mM increase, it is important to consider long-term treatment goals. While novel therapies have generated considerable excitement, HD IL-2 has the longest experience with durable response and survival in mM and mRCC. Recent registry data have confirmed that HD IL-2 remains an essential component of the therapeutic armamentarium for mRCC and mM. These data reinforce clinical trial findings that HD IL-2 produce durable CRs that are apparent soon after treatment and can last for decades. Use of HD IL-2 can offer patients significant clinical benefit with a short duration of treatment while preserving the opportunity for additional therapies if needed.
As strategies are being developed to determine predictive markers, both pathologic and clinical, the use of HD IL-2 in cancer immunotherapy continues to evolve, as does its role in combination with other immunemodulating agents. These strategies include combined treatment with vaccines, antibody therapies to block inhibitory pathways, and adoptive cell transfer of T cells. While there remains a need for the development of biomarkers to identify IL-2 responders, new approaches have the promise to increase the number of patients who may derive benefit from this treatment. Results from these clinical strategies would help develop novel combined treatment approaches.
While several matters remain to be resolved in these combined and sequential approaches, including the optimal dose and timing of therapies, and toxicity of combined approaches, these new approaches continue to keep HD IL-2 an important part of the treatment armamentarium in mRCC and mM.