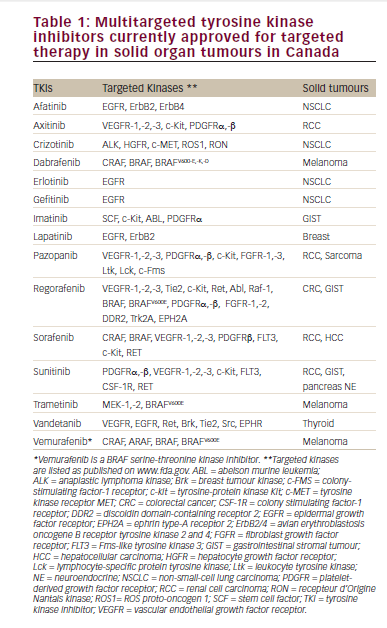
Q. What are the currently approved treatment options for newly diagnosed patients with metastatic renal cell carcinoma (RCC)?
The currently approved therapies are of two different types: targeted therapy and immunotherapy. Between 2005 and 2014 the backbone of therapy in RCC was anti-angiogenic therapy and in 2015 immune therapy in the form of immune checkpoint inhibitors began to get US Food and Drug Administration (FDA) approval. Currently there are about nine agents/regimens approved for RCC.
Q. How have the emergence of immune checkpoint inhibitors and targeted therapies affected the treatment paradigm for first-line treatment of RCC?
The treatment efficacy has improved remarkably and there is now the hope of long-term remission in metastatic RCC.
Q. What was the most exciting data to emerge in the past year supporting the use of these agents?
The most exciting data is from a study evaluating the combination of ipilimumab and nivolumab, which showed a long (>30 months) median overall survival. So the life expectancy in advanced RCC has about tripled in the last 20 years.1
Q. What patient factors would influence your choice of first-line agent?
There is an International Metastatic Renal-cell Carcinoma Database Consortium (IMDC) model and I use the risk stratification from this when I evaluate patients.2 Now that we have potential options such as bevacizumab and atezolizumab in combination showing efficacy in programmed death-ligand 1 (PDL-1) positive patients, this test may be worthwhile getting. Patients with bone metastases, symptomatic or rapidly progressive aggressive disease will likely need cabozantinib, a vascular endothelial growth factor (VEGF) and c-Met inhibitor tyrosine kinase inhibitor with an 80% chance of shrinking tumors.
Q: Is there still a place for high-dose interleukin (IL) in the treatment paradigm?
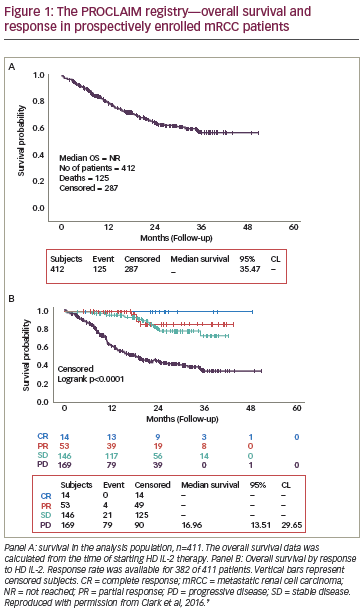
I still evaluate for front-line interleukin as this agent still has the longest track record of showing long-term remissions with 20-year follow up. Only about 10% of my patients with metastatic RCC will be candidates for IL-2.